As described in part 1 of this 3-part series (“Etiology and Diagnosis of Vision Degrading Myodesopsia,” available at https://www.retinalphysician.com/issues/2022/june-2022/etiology-and-diagnosis-of-vision-degrading-myodeso ), the diagnosis of vision degrading myodesopsia (VDM), characterized by clinically significant vitreous floaters, is established on the basis of measured increases in vitreous density (by quantitative ultrasonography) and quantified degradation in contrast sensitivity function (CSF).1-3 Once the diagnosis of VDM is established and therapy is deemed appropriate, the next challenge is to cure the problem. The following article reviews surgical intervention with vitrectomy, while the third article in this series will review nonsurgical approaches.
PARS PLANA VITRECTOMY
In 2002, Delaney et al reported a series of 39 eyes with vitreous opacities that underwent YAG laser treatment with poor results.4 In 15 of the eyes, subsequent pars plana vitrectomy achieved full resolution of symptoms in 93.5%, leading the authors to conclude that vitrectomy offered superior results. In 2011 Schulz-Key et al used a nonstandardized questionnaire in 73 patients, concluding that 88% were satisfied with the results (mean follow-up 37 months).5 However, there was a 6.8% incidence of retinal detachment and a 60% incidence of cataracts. In a 2013 study of 110 eyes that had 20-gauge vitrectomy in half and 23-gauge in the other half, de Nie et al used the standardized National Eye Institute Visual Function Questionnaire (VFQ-25) to assess outcomes and found that 85% were either “satisfied” or “very satisfied” with the results.6 In 2016, Milston et al reviewed several studies (728 eyes) of vitrectomy for VDM, concluding that there was considerable evidence supporting the efficacy of vitrectomy.2 However, visual function was not analyzed or improved in the majority of these studies. The first reports of improved vision after vitrectomy for VDM appeared in 2014, when Mason et al observed increased visual acuity (VA) by one line or more, albeit in only 37.5% of 168 cases.7 An extensive review by Ivanova et al in 2016 found that of all available treatment options, pars plana vitrectomy offered the best solution to cure vitreous floaters, despite potential associated risks.8
SURGICAL TECHNIQUE OF LIMITED VITRECTOMY
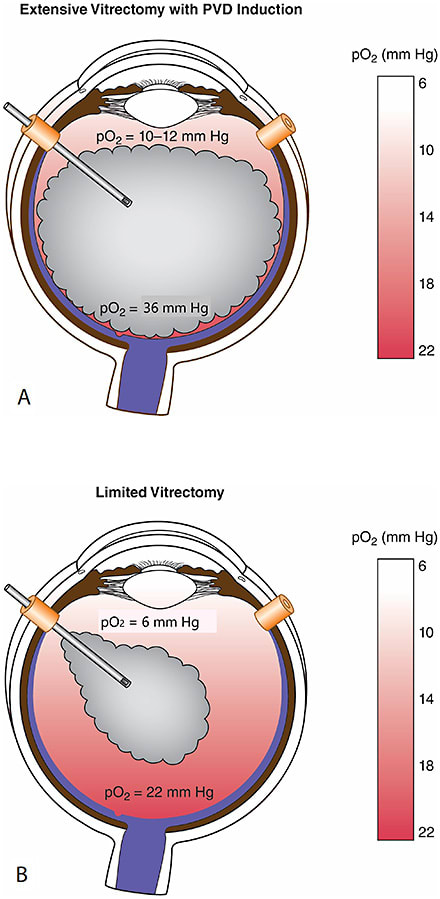
For the past decade we have performed limited vitrectomy in the Newport Bay Ambulatory Surgery Center with retrobulbar anesthesia and 25-gauge instruments, at all times sutureless. Highly beveled incisions were used for entry and a nonhollow probe was employed to remove self-retaining cannulas. In younger patients without a posterior vitreous detachment (PVD), no surgical PVD was induced. To mitigate cataract formation, 3-4 mm of gel vitreous was left intact behind the lens because of the presence of antioxidants in native vitreous. The underlying theory relates to elevated intravitreal oxygen levels following extensive vitrectomy (Figure 1), which would promote cataracts.9,10
BENEFITS
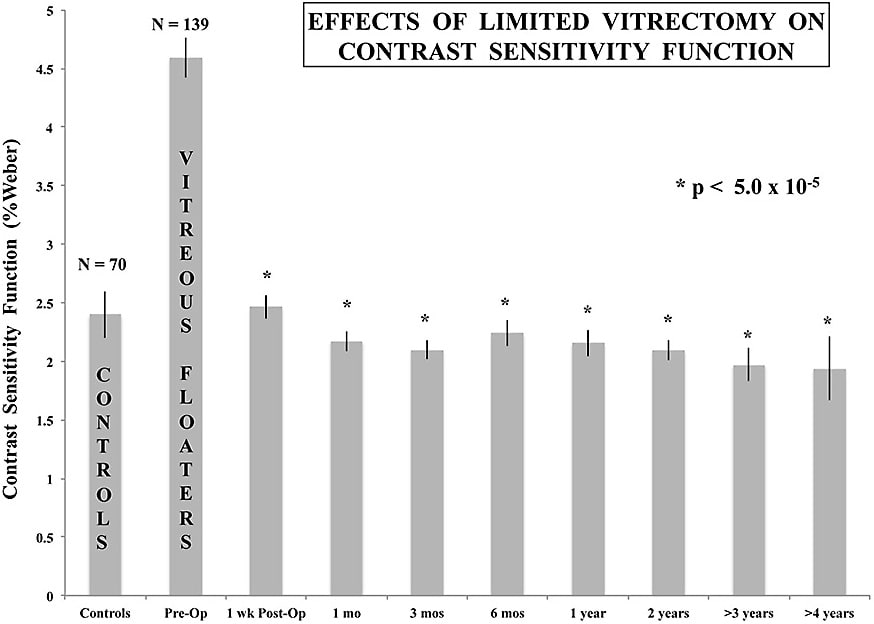
In 2014, Sebag et al reported that in 76 eyes with VDM, preoperative CSF was degraded by 67% compared to controls (P<0.013) and the VFQ-25 composite score was 28.3% lower in patients with floaters than age-matched controls (P<.001).11 Following limited vitrectomy, VFQ-25 improved by 29.2% (P<.001) and CSF normalized in each case at 1 week (P<.01), 1 month (P<.003), and 3 to 9 months (P<.018) postoperatively. More recently, in the largest single-center study reported to date, 195 cases of VDM were followed for an average of 32.6 months (50 cases for more than 4 years) after limited vitrectomy.12 Vitreous echodensity decreased by 94.1% (P<.0001), visual acuity showed a statistically significant improvement (P<.0001), CSF (which preoperatively was 91.3% worse in patients with VDM vs controls; P<.0001) normalized at 1, 3, 6, 12, 24, 36, and 48 months postoperatively (P<.00005 for each; Figure 2). VFQ-25 results improved by a modest 19.3% (P<.0001), likely due to discriminatory limitations in the questionnaire itself.13
RISKS
Although these studies showed strong evidence of efficacy, there has historically been debate about whether the benefits justify the potential risks. In 2011, Schulz-Key et al performed 20-gauge vitrectomy and reported a 6.8% incidence of retinal detachment and a 60% incidence of cataracts.6 In the same year, Tan et al used both 20-gauge and 23-gauge instruments with PVD induction during surgery, finding that 30% of cases were associated with retinal tears.14 Cataracts developed in 50% of cases, but follow-up averaged only 10.1 months, and more patients probably developed cataracts later, consistent with the findings of Schulz-Key et al.6 In 2013, de Nie et al found a 10.9% incidence of retinal detachment, which is significant.7 In all of these studies, PVD induction was frequently performed, which is very likely a major contributor to retinal breaks (Figure 3).
MITIGATING RISKS
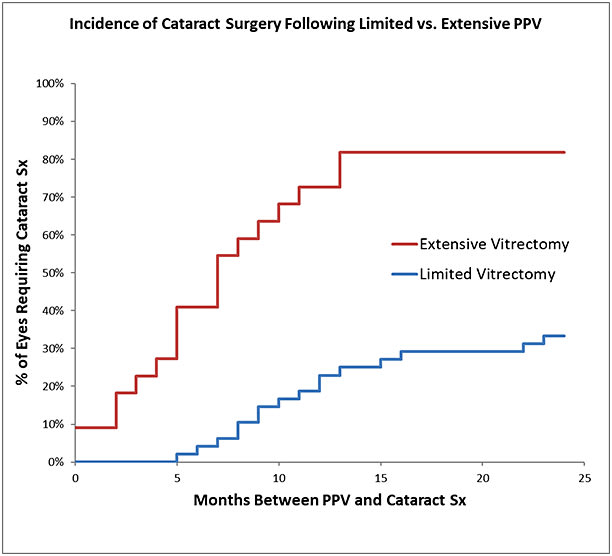
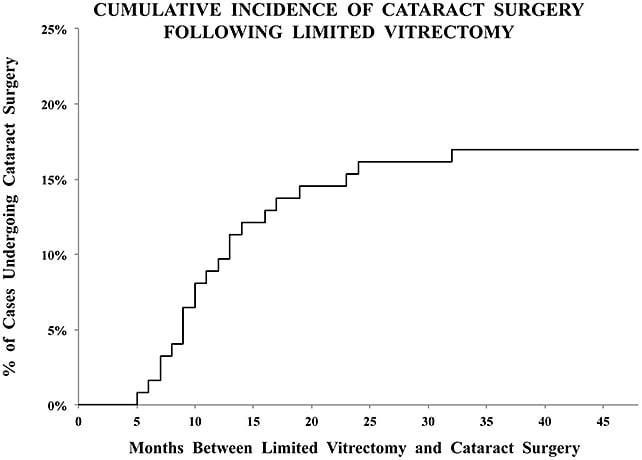
Limited vitrectomy is performed with small-gauge instruments and no surgical PVD induction to avoid iatrogenic retinal breaks and postoperative retinal detachments. Careful preoperative examination to rule out peripheral retinal pathology and prophylactic treatment (performed in almost one-quarter of eyes by Sebag et al14) further ensure safety. To avoid cataract, retrolental gel vitreous is left intact, because vitreous contains powerful antioxidants (Figure 1).15 Initial experience in 76 cases found that only 23.5% of cases required cataract surgery (follow-up 17.5 months; no retinal detachments).11 A separate study compared limited vitrectomy to extensive vitrectomy, finding a lower incidence of postoperative cataract surgery (35% at 24 months following limited vitrectomy vs 87% after extensive vitrectomy; P<.0001) (Figure 4).16 The time to cataract surgery was also twice as long after limited vitrectomy (P<.002). A larger series (195 cases) followed for an average of 32.6 months found that cataract surgery was performed in 16.9% of cases, on average 13.1 months after vitrectomy. The mean age of these patients was 64 years, and none was younger than 53 years old (Figure 5).12
There were no cases of endophthalmitis, acute vitreous hemorrhage occurred in 1% (cleared spontaneously), retinal tears were seen postoperatively in 1.5%, and retinal detachments occurred in 1.5% (2 weeks, 10 months, 14 months postoperative). All were treated successfully with 1 procedure. Among 17,615 vitrectomies for vitreous opacities from the IRIS registry, only 2.6% of patients required subsequent retinal detachment repair.17 These results clearly established limited vitrectomy as a safe and effective treatment (Table 1).
| BENEFITS | RISKS |
|---|---|
A recent multicenter study by the European Vitreoretinal Society found that in 678 cases with symptomatic floaters, pars plana vitrectomy by multiple surgeons with different techniques resulted in high patient satisfaction and a relatively low rate of severe complications.18 The authors concluded that surgery may be safer with core vitrectomy, no PVD induction, and moderately high cut rates (>1,500 cuts per minute).
An ever-increasing important consideration in health care is cost and value. A recent study quantified the cost-effectiveness of limited vitrectomy for VDM, finding that in addition to increased postoperative VA (P<.00001), normalized CSF (P<.00001), and improved VFQ-39 scores (P<.00001), the incremental value gain was 2.38 quality-adjusted life years (QALYs).19 The average cost–utility ratio (2018 US real dollars) was $1,574/QALY for limited vitrectomy, which is superior to cataract surgery ($2,262/QALY), amblyopia therapy ($2710/QALY), and retinal detachment repair ($45,304/QALY).20
FUTURE CONSIDERATIONS
As we look to the future, the large instruments originally built in the 1970s by Jean Marie Parel have been miniaturized to the current 25-gauge and 27-gauge sizes, with development of 29-gauge and 30-gauge vitrectomy instruments for pediatric vitreoretinal surgery.21 Smaller gauge instruments might be particularly suited for treating vitreous floaters, because extensive peripheral vitrectomy is not required for VDM surgery. Indeed, Khan et al studied 69 cases undergoing 27-gauge vitrectomy (subconjunctival anesthesia) for vitreous floaters; these patients had no complications other than slight elevation of intraocular pressure (IOP) at 1 year (P<.01), which was still within the normal range.22
CONCLUSIONS
Advances in surgical technique and the development of objective quantitative outcome measures have made limited vitrectomy a treatment modality which offers patients a low risk profile as well as a cost-effective way to experience objective improvement in vitreous structure and visual function.23 This has helped to promote acceptance of vitrectomy as a reasonable therapeutic option in the management of VDM. In fact, the 2021 Global Trends in Retina Survey of the American Society of Retinal Specialists (ASRS) determined that 85% of the 893 respondents perform vitrectomy for floaters.24 RP
REFERENCES
- Mamou J, Wa CA, Yee KMP, et al. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Investig Ophthalmol Vis Sci. 2015;56(3):1611-1617. doi:10.1167/iovs.14-15414
- Milston R, Madigan MC, Sebag J. Vitreous floaters: etiology, diagnostics, and management. Surv Ophthalmol. 2016;61(2):211-227. doi:10.1016/j.survophthal.2015.11.008
- Garcia GA, Khoshnevis M, Yee KMP, Nguyen-Cuu J, Nguyen JH, Sebag J. Degradation of contrast sensitivity function following posterior vitreous detachment. Am J Ophthalmol. 2016;172:7-12. doi:10.1016/j.ajo.2016.09.005
- Delaney Y, Oyinloye A, Benjamin L. Nd:YAG vitreolysis and pars plana vitrectomy: surgical treatment for vitreous floaters. Eye. 2002;16(1):21-26. doi:10.1038/sj/EYE/6700026
- Schulz-Key S, Carlsson JO, Crafoord S. Long-term follow-up of pars plana vitrectomy for vitreous floaters: complications, outcomes, and patient satisfaction. Acta Ophthalmol. 2011;89(2):159-165. doi:10.1111/j.1755-3768.2009.01682.x
- De Nie KF, Crama N, Crama MAD, Klevering BJ, Boon CJF. Pars plana vitrectomy for disturbing primary vitreous floaters: clinical outcome and patient satisfaction. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(5):1373-1382. doi:10.1007/s00417-012-2205-3
- Mason JO, Neimkin MG, Friedman DA, Feist RM, Thomley ML, Albert MA. Safety, efficacy, and quality of life following sutureless vitrectomy for symptomatic vitreous floaters. Retina. 2014;34(6):1055-1061. doi:10.1097/IAE.0000000000000063
- Ivanova T, Jalil A, Antoniou Y, Bishop PN, Vallejo-Garcia JL, Patton N. Vitrectomy for primary symptomatic vitreous opacities: an evidence-based review. Eye. 2016;30(5):645-655. doi:10.1038/eye.2016.30
- Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 139(2):302-10, 2005. doi:10.1016/j.ajo.2004.09.046
- Beebe DC, Holekamp NM, Siegfried C, Shui YB: Vitreo-retinal influences on lens function and cataract. Trans Roy Soc Lond B Biol Sci. 2011;366(1568):1293-1300. doi:10.1098/rstb.2010.0228
- Sebag J, Yee, KMP, Huang L, Wa C, Sadun AA. Vitrectomy for floaters: prospective efficacy analyses and retrospective safety profile. Retina. 2014; 34(5):1062-1068. doi:10.1097/IAE.0000000000000065
- Sebag J, Yee KMP, Nguyen JH, Nguyen-Cuu J. Long-term safety and efficacy of limited vitrectomy for vision degrading myodesopsia resulting from vitreous floaters. Ophthalmol Retina. 2018;2(9):881-887. doi:10.1016/j.oret.2018.03.011
- Nguyen-Cuu J, Nguyen E, Yee KMP, Nguyen J, Sebag J. Self-administered questionnaires correlate with visual function and vitreous structure in patients with vitreous floaters. Invest Ophthalmol Vis Sci. 2017;58(8):1346.
- Tan HS, Mura M, Lesnik Oberstein SY, Bijl HM. Safety of vitrectomy for floaters. Am J Ophthalmol. 2011;151(6):995-998. doi:10.1016/j.ajo.2011.01.005
- Ankamah E, Sebag J, Ng E, Nolan JM. Vitreous antioxidants, degeneration, and vitreo-retinopathy: exploring the links. Antioxidants. 2019;9(1):7.
- Yee KMP, Tan S, Lesnik Oberstein SY, et al. Incidence of cataract surgery after vitrectomy for vitreous opacities. Ophthalmol Retin. 2017;1(2):154-157. doi:10.1016/j.oret.2016.11.012
- Sebag J. Vitrectomy for vision degrading myodesopsia. Ophthalmol Retina. 2021;5(1):1-3. doi:10.1016/j.oret.2020.08.013
- Zeydanli EO, Parolini B, Ozdek S, et al. Management of vitreous floaters: an international survey: the European VitreoRetinal Society Floaters study report. Eye (Lond). 2020;34(5):825-834. doi:10.1038/s41433-020-0825-0
- Rostami B, Nguyen-Cuu J, Brown G, Brown M, Sadun AA, Sebag J. Cost-effectiveness of limited vitrectomy for vision-degrading myodesopsia. Am J Ophthalmol. 2019;204:1-6. doi:10.1016/j.ajo.2019.02.032
- Brown MM, Brown GC, Sharma S, Garrett S. Evidence-based medicine, utilities, and quality of life. Curr Opin Ophthalmol. 1999;10(3):221-226. doi:10.1097/00055735-199906000-00012
- Binder S, Wimpissinger B, Kellner L. Current clinical data and future for small gauge vitreoretinal surgery. In: Rizzo S, Patelli F, Chow D, eds. Vitreo-Retinal Surgery. Springer; 2009:213-222.
- Khan MA, Kuley A, Riemann CD, et al. Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior segment disease. Ophthalmology. 2018;125(3):423-431. doi:10.1016/j.ophtha.2017.09.013
- Sebag J. Vitreous and vision degrading myodesopsia. Prog Retin Eye Res. 2020;79:100847. doi:10.1016/j.preteyeres.2020.100847
- Hahn P, Eliott D, eds. 2021 Global Trends in Retina Survey. American Society of Retina Specialists; 2021. https://www.asrs.org/content/documents/_asrs-2021-pat-survey-for-website.pdf








