Intravitreal injections (IVI) are widely performed in the practice of retinal care. Most syringes are lubricated with silicone oil (SiO) to allow the piston to glide more easily (Figure 1A). Silicone oil has been considered an inert and usually harmless material, and consequently it has been used for a long time in heart valves, breast implants, and as a tamponade for retinal detachment surgery.1-2
Despite the utility of silicone oil, there is evidence that it might have some detrimental effects to the eye, including the development of myodesopsia (Figure 1B). “Floaters” of different sizes and shapes can become annoying, leading to the need for surgery in some patients. Although a minority of patients experience floaters, it has been recently acknowledged that the prevalence of this problem is higher than previously believed.3,4 Additionally, a survey carried out by the American Society of Retina Specialists has shown that about 5% of their US members have performed vitrectomy to treat patients with symptomatic floaters, and 2% have seen patients seeking legal action.5 Awareness of this association becomes more important in light of the large number of injections performed worldwide every year.
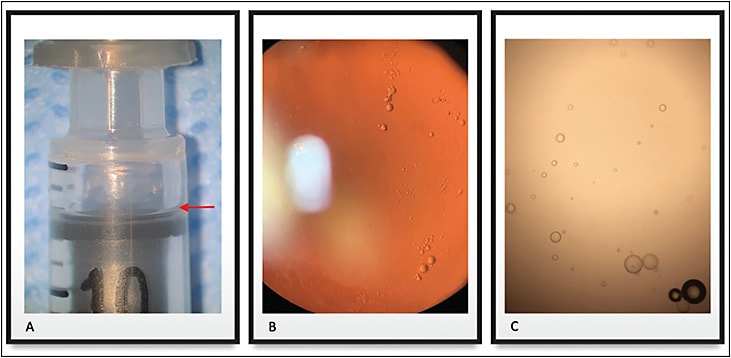
Most commercially available syringes are coated with silicone oil, and research has examined complications resulting from intravitreal injections using siliconized syringes.1 After a clinical observation of noninfectious endophthalmitis associated with the syringe in a case series, we found that the SiO is especially released by agitation, such as when the syringe is flicked or tapped (Figure 1C).1 Research by Kim et al also supports these findings.6
In addition to lab-based research, a clinical trial assessed whether agitation of a siliconized syringe could lead to the detection of inflammatory cells in the anterior chamber of the eye following an intravitreal injection of aflibercept.7 Subjects undergoing an IVI before vitrectomy for proliferative diabetic retinopathy (DR) were injected with the drug 48 hours preoperatively. The control group received the injection without agitation, while the intervention group was injected with a siliconized syringe that had been agitated by flicking. The primary endpoint was the presence of anterior chamber (AC) cells, assessed at baseline and 48 hours later at the slit lamp by a masked grader following the International Uveitis Study Group (IUSG) clinical classification of uveitis. Neither patients nor injecting or grading physicians were aware of the assigned group (agitation or no agitation). All eyes were free of AC cells, hyperemia, and pain at baseline. Although 10% and 20% of the eyes in the no-agitation groups of the siliconized and SiO-free syringes, respectively, showed AC cells, roughly 80% of the eyes treated with an agitated siliconized syringe showed such findings, and this difference was statistically significant. The agitation group of the SiO-free syringes showed AC cells in 36% of the eyes, but this difference was not statistically significant. This clinical trial discloses the potential role of agitation in the development of inflammation after IVIs of aflibercept using a siliconized syringe. Additional studies with different syringes and drugs should be conducted to obtain a broader and more consistent perspective.
BASIC SCIENCE BACKGROUND
It is well known that agitation of prefilled syringes to expel air at the moment of drug preparation, or even during transportation, not only leads to SiO release but also to protein aggregation, which might ultimately lead to increased immunogenicity.8 Studies have shown that SiO microdroplets can act as an adjuvant to promote a break in immunologic tolerance and induce antibody response.8 An increased secretion of several innate cytokines from human peripheral blood mononuclear cells, and also in the plasma concentrations of antidrug antibodies, were found when a siliconized syringe was agitated in comparison to both an unagitated syringe and to an agitated SiO-free syringe.8
Protein aggregates can be formed under different situations prior to IVI of the drug, and they can be further induced by SiO release from syringes.9-11 Various factors can contribute to SiO release from the inner cylinder wall, protein denaturing, or both.1,9-11
Shipping, handling, and temperature of storage of anti-VEGF medications and syringes are some of these factors.1,9-11 Freeze-thawing that may occur during shipping has been demonstrated to significantly increase SiO microdroplet release in bevacizumab solution, along with increased protein particle levels.10 In contrast, high temperatures may also promote particle formation, protein unfolding and aggregation.12,13 Light exposure has been shown to increase particulate counts in protein solutions.10
An immune response can be originated from SiO–protein complex formation and protein aggregates.14,15 Proteins from the drug solution may interact with SiO and cause protein modification, altering the proteins and creating aggregates (Figure 2). Even microscopic SiO droplets have been shown to have the potential to create these potentially immunogenic protein complexes. Some studies have shown that an immunogenic reaction might be secondary to the interaction of micron-sized and submicron-sized SiO droplets that form SiO-protein complexes with monoclonal antibodies.8,14 Imaging techniques have also confirmed that micron-sized and submicron-sized SiO droplets are able to cause protein complexes and aggregates.16 These studies suggest that even small amounts of SiO may interact with the anti-VEGF molecules, and this may result in potentially immunogenic alterations of these antibodies.
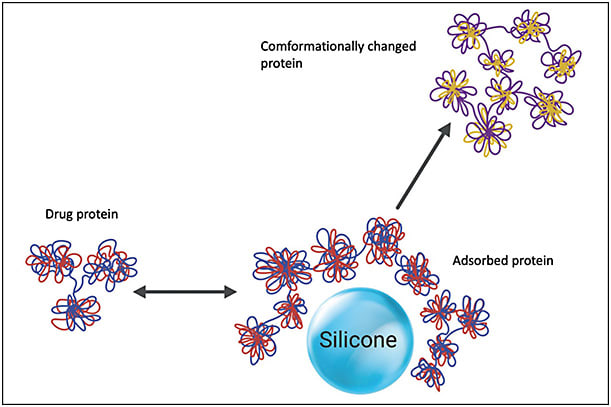
Most studies have shown variable aggregation depending on the protein structure, even though SiO-induced protein aggregation may theoretically affect all proteins. Further investigation is necessary as to whether it happens with some anti-VEGF agents more often than with others.
POTENTIAL INVOLVEMENT IN INFLAMMATION
Silicone oil microdroplets may lead to an antibody response against a self-protein, and the capability of these microdroplets to behave as adjuvants and amplify antibody reaction against injected drug proteins may be related to these proteins’ inherent immunogenicity.15 A lab study showed that formulations of recombinant murine growth hormone that included SiO microdroplets could decrease the immunologic tolerance to a recombinant self-protein when administered daily to mice.17 The authors also found that the frequency of the injection played a role.17 The inherent protein immunogenicity may determine the adjuvant potency of SiO, with external proteins inducing more intense effects. Another report found that adsorption of proteins to SiO could change the protein structure (Figure 2).18 Furthermore, the presence of SiO microdroplets acted as an adjuvant to enhance the immune reaction. This complementary effect did not develop in the setting of protein and SiO injected separately, leading to a hypothesis that the adsorption of protein to SiO or the simultaneous presence of both should be critical for the expression of this suspected adjuvant effect.18
An IVI of a therapeutic agent associated with aggregated protein is a reason for concern, because protein aggregates can induce an immune response.19 An immunologic response develops after an antigen stimulation by provoking naïve B and T lymphocytes and comprises an interplay adjustment between antigen and lymphocytes. The altered immune response to protein formulations that have SiO microdroplets is probably associated with a T-cell-dependent B cell activation process related to IgG1 production.18,20,21 Therefore, the T-cell-dependent immune reaction against a foreign protein antigen could explain the IgG expression with consecutive development of intraocular inflammation following intravitreal injection of therapeutic proteins in conjunction with SiO.22
CONCLUSIONS
It is important that retina specialists doing IVI be aware that silicone oil might be released by most commercially available syringes, leading to a complaint of floaters. Additionally, many lab-based studies unrelated to ophthalmology have shown a clear association between agitation of a siliconized syringe and protein aggregation, leading to an immunologic response. Finally, some clinical studies in ophthalmology have suspected this interaction might play a role in the development of inflammation after an intravitreal injection. More research should be further developed to provide a better understanding of this subject. RP
REFERENCES
- Melo GB, Cruz NFSD, Emerson GG, et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog Retin Eye Res. 2021;80:100862. doi:10.1016/j.preteyeres.2020.100862
- Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4-8. doi:10.1016/j.jaut.2010.07.003
- Thompson JT. Prospective study of silicone oil microdroplets in eyes receiving intravitreal anti-vascular endothelial growth factor therapy in 3 different syringes. Ophthalmol Retina. 2021;5(3):234-240. doi:10.1016/j.oret.2020.07.021
- Melo GB, Dias Junior CS, Morais FB, et al. Prevalence of silicone oil droplets in eyes treated with intravitreal injection. Int J Retina Vitreous. 2019;5:34. doi:10.1186/s40942-019-0184-9
- Stone TW, ed. ASRS 2018 preferences and trends membership survey. Presented at: American Society of Retina Specialists annual meeting; July 20-25, 2018; Chicago, IL. Accessed May 18, 2022. https://www.asrs.org/content/documents/_2018-pat-survey-results-for-website.pdf
- Kim NA, Kim DJ, Jeong SH. Do not flick or drop off-label use plastic syringes in handling therapeutic proteins before administration. Int J Pharm. 2020;587:119704. doi: 10.1016/j.ijpharm.2020.119704
- Cruz NFS, Polizelli MU, Muralha FP, et al. Ocular inflammation after agitation of siliconized and silicone oil-free syringes: a randomized, double-blind, controlled clinical trial. Int J Retina Vitreous. 2022, in press.
- Krayukhina E, Yokoyama M, Hayashihara KK, et al. An assessment of the ability of submicron- and micron-size silicone oil droplets in dropped prefillable syringes to invoke early- and late-stage immune responses. J Pharm Sci. 2019;108(7):2278-2287. doi:10.1016/j.xphs.2019.02.002
- Krayukhina E, Tsumoto K, Uchiyama S, Fukui K. Effects of syringe material and silicone oil lubrication on the stability of pharmaceutical proteins. J Pharm Sci. 2015;104(2):527-535. doi:10.1002/jps.24184
- Liu L, Ammar DA, Ross LA, Mandava N, Kahook MY, Carpenter JF. Silicone oil microdroplets and protein aggregates in repackaged bevacizumab and ranibizumab: effects of long-term storage and product mishandling. Invest Ophthalmol Vis Sci. 2011;52(2):1023-1034. doi: 10.1167/iovs.10-6431
- Torisu T, Maruno T, Yoneda S, et al. Friability testing as a new stress-stability assay for biopharmaceuticals. J Pharm Sci. 2017;106(10):2966-2978. doi:10.1016/j.xphs.2017.05.035
- Ahrer K, Buchacher A, Iberer G, Jungbauer A. Thermodynamic stability and formation of aggregates of human immunoglobulin G characterised by differential scanning calorimetry and dynamic light scattering. J Biochem Biophys Methods. 2006;66(1-3):73-86. doi:10.1016/j.jbbm.2005.12.003
- Freire E, Schon A, Hutchins BM, Brown RK. Chemical denaturation as a tool in the formulation optimization of biologics. Drug Discov Today. 2013;18(19-20):1007-1013. doi:10.1016/j.drudis.2013.06.005
- Uchino T, Miyazaki Y, Yamazaki T, Kagawa Y. Immunogenicity of protein aggregates of a monoclonal antibody generated by forced shaking stress with siliconized and nonsiliconized syringes in BALB/c mice. J Pharm Pharmacol. 2017;69(10):1341-1351. doi:10.1111/jphp.12765
- Chisholm CF, Nguyen BH, Soucie KR, Torres RM, Carpenter JF, Randolph TW. In vivo analysis of the potency of silicone oil microdroplets as immunological adjuvants in protein formulations. J Pharm Sci. 2015;104(11):3681-3690. doi: 10.1002/jps.24573
- Probst C. Characterization of protein aggregates, silicone oil droplets, and protein-silicone interactions using imaging flow cytometry. J Pharm Sci. 2020;109(1):364-374. doi:10.1016/j.xphs.2019.05.018
- Chisholm CF, Baker AE, Soucie KR, Torres RM, Carpenter JF, Randolph TW. Silicone oil microdroplets can induce antibody responses against recombinant murine growth hormone in mice. J Pharm Sci. 2016;105(5):1623-1632. doi:10.1016/j.xphs.2016.02.019
- Chisholm CF, Soucie KR, Song JS, et al. Immunogenicity of structurally perturbed hen egg lysozyme adsorbed to silicone oil microdroplets in wild-type and transgenic mouse models. J Pharm Sci. 2017;106(6):1519-1527. doi:10.1016/j.xphs.2017.02.008
- Fradkin AH, Carpenter JF, Randolph TW. Immunogenicity of aggregates of recombinant human growth hormone in mouse models. J Pharm Sci. 2009;98(9):3247-3264. doi: 10.1002/jps.21834
- Stevens TL, Bossie A, Sanders VM, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334(6179):255-258. doi:10.1038/334255a0
- Filipe V, Que I, Carpenter JF, Lowik C, Jiskoot W. In vivo fluorescence imaging of IgG1 aggregates after subcutaneous and intravenous injection in mice. Pharm Res. 2014;31(1):216-227. doi: 10.1007/s11095-013-1154-9
- Anderson WJ, da Cruz NFS, Lima LH, Emerson GG, Rodrigues EB, Melo GB. Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review. Int J Retina Vitreous. 2021;7(1):37. doi:10.1186/s40942-021-00307-7








