Optical coherence tomography angiography (OCTA) has become increasingly available and used as an imaging modality in the past several years. The noninvasive nature of OCTA is a major benefit over more invasive imaging procedures such as fluorescein angiography (FA). Even though OCTA does not provide some of the information that FA does, the information that it does provide is valuable and provides insight into retinal vascular diseases.1
Optical coherence tomography angiography captures the path of retinal microvasculature by using light reflected off the moving red blood cells. It can show the path of the blood flow in relation to the retinal layers seen on OCT and provides 3D information regarding the retinal vascular plexuses. Pathologic findings that have been well visualized by OCTA include enlarged or irregular foveal avascular zones, microaneurysms, choroidal neovascularization2,3, and retinal ischemia. Thus, disease entities commonly imaged with OCTA include diabetic retinopathy,4 sickle cell retinopathy,5 and age-related macular degeneration,6 to name a few. Pathologies requiring identification of time-dependent biomarkers, such as perfusion timing, or fluorescein dye leakage, may not benefit as much from OCTA.
As with any imaging modality, interpretation of the images is key. This article assumes basic knowledge of the OCTA layers and will focus more on imaging artifacts and common pitfalls, as well as some tips for do-it-yourself imaging and critical analysis.
COMMON ARTIFACTS AND PITFALLS
The following common artifacts will be discussed: blink, motion, out of focus, media opacity, shadowing, hypertransmission, incorrect segmentation, and projection.7
- Blink, or dry eye, artifact presents as gradual blurring and loss of signal in the direction that the scans are captured (Figure 1). This can be remedied by applying artificial tears before imaging.

Figure 1. Blink artifact shown on the OCTA superficial vascular complex (A) and deep vascular complex (B). Note the sudden transition from the sharp vasculature toward the top of the images, then the gradual reconstitution of the sharpness toward the bottom. - Motion artifacts present as sudden, abrupt disruptions in the image (Figure 2). These occur when the patient suddenly moves their eyes. On review of the OCT B-scans, some of the images may be inverted due to patient movement.
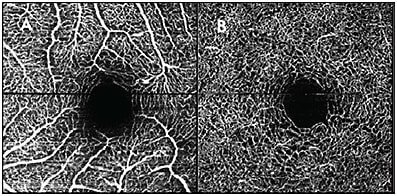
- Focus, if not achieved, can cause artifacts to occur (Figure 3). Blurry vasculature may be interpreted as increased vessel caliber or it may wash out the finer microvasculature. Optimize the focus by using the appropriate settings to account for eye length. Patients with high astigmatism may be best imaged through their glasses.
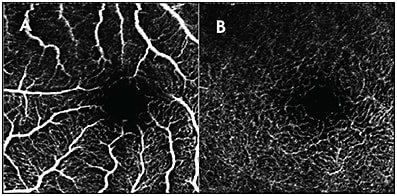
- Media opacities can block signal and be misinterpreted as flow voids. The OCT B-scans may show as sharply demarcated spots without any blood flow through all layers. Often, the imager will notice that these spots are mobile and can recognize that they are due to floaters. If a repeat image is obtained, the areas without flow will change, thus providing a clue that a media opacity is blocking the light.
- Shadowing from retinal opacification, such as in retinal scars or edema, is another common artifact that can be misinterpreted as a flow void. Review of the OCT B-scans through this area will reveal the hyperreflective retinal layers causing this artifact.
- Hypertransmission of signals is the opposite of shadowing. This is caused by increased OCT signal, for example due to RPE defects or from exudates, and can be misinterpreted as increased flow. These signals are often in a nonvascular pattern and can be identified by examining the OCT B-scans in the areas of signal hypertransmission.
- Segmentation artifacts may occur if the retinal layers are not identified correctly. This occurs more commonly when there is retinal pathology that deviates from the usual structure of the healthy adult retina. Examples in which this may occur include the retinas of premature infants8 and in retinoblastoma9 (Figure 4).
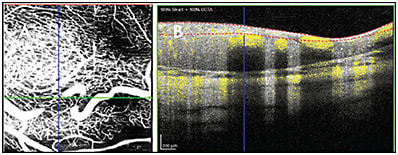
- Projection artifacts can be caused by the more superficial retinal vasculature, and this creates the appearance of vasculature in the deeper retinal layers. These artifacts can be removed by various projection-resolved algorithms so that they do not mask the vasculature in the deeper retinal layers.10,11
DIY IMAGING TIPS
Optimal imaging helps avoid some artifacts that confound the analysis of OCTA images, in particular artifacts due to dry eyes, motion, and being out of focus. To achieve high-quality images and avoid as many of the common artifacts and pitfalls as possible, preparation, positioning, and patient coaching are important. The following tips will focus on optimizing OCTA imaging using a tabletop machine in clinic.12
Tip #1: Set the Scene
The process of achieving high-quality images begins prior to the patient entering the room for imaging. Especially in patients for whom attention span or cooperation is limited (for example, children), the amount of time they will graciously grant should not be spent on waiting for the computer to turn on or the program to launch. Have the patient’s name, date of birth, and other pertinent information easily accessible and ready to quickly enter into the system; better yet, enter it before they arrive. If there is a particular lesion of interest, the physician should communicate it to the technician performing the imaging.
Tip #2: Dilate and Lubricate
Pharmacologic dilation, although not necessary, is helpful to achieve optimal imaging and allow for more wiggle room in alignment and focus. Dimming the room lights to allow for physiologic dilation is also helpful, but adjustments to room lighting alone may not be adequate depending on the patient. Artificial tears to lubricate the patient’s eyes can help avoid artifacts from blinking and dry eyes and improve image quality. In patients who will tolerate artificial tears, consider putting these in prior to imaging, especially in cases where the number of attempts or amount of time to be spent on imaging is limited. The higher the quality of the images, the faster the OCTA images will be acquired. Avoid artificial tears in children who might be distressed by eye drops.
Tip #3: Make the Machine Work for You
Settings may also need to be adjusted for patients who fall outside of the average measurements for axial length, refractive error, corneal curvature, or astigmatism. A common cause of images being out of focus is high astigmatism. To help fix this, as mentioned before, consider imaging the patient through their glasses.
Tip #4: Make It Fun!
When treating children, establishing rapport with them and their parents or guardians is important. Turning the imaging process into a fun game can improve the experience. Describe the handles under the headrest as “motorcycle handles,” and have the child pretend to ride a motorcycle. Tell them the goal is to stare down the bright light, and to hold as still as they can as you count to 10 (or 20). Consider also imaging their parents or guardians or older, more cooperative siblings first to show them that the process is nonthreatening.
Tip #5: Comfort and Stability Are Key
Positioning the patient so that they are comfortable and stable will improve image quality and help avoid motion artifacts. In adults, adjust the chair, tabletop and chinrest to the appropriate level. In children, depending on their height, consider the alternative option of standing on a step stool rather than sitting in the chair. If there is a family member or extra person in the room, have them place their hand at the back of the patient’s head for as a gentle reminder to keep the head still.
Tip #6: Fixate
Good fixation not only maximizes image quality and prevents motion artifacts, but also allows faster acquisition of images and thus demands less imaging time from the patient. Remove distracting targets that may compete for the patient’s attention. A common distraction is the computer screen itself; tilt the screen so that it is not visible to the patient. In patients who have difficulty fixating while the eye is being imaged, switch to an external fixation target. External fixation targets can be the light attached to the tabletop machine, an adhesive note on the wall behind the imager, or a cartoon character on a smartphone being held by a parent. Coaching the patient throughout imaging to continue looking at the target can also be helpful.
DIY ANALYSIS TIPS
Analysis and interpretation of OCTA images can often be quickly accomplished by looking at the images of the en face vascular layers that have been automatically segmented and processed by the OCTA software (Figure 4). In healthy adult eyes, for which most of the image processing software has been written and trained to analyze, such a method of reading is sufficient. However, for eyes with pathological findings, manual segmentation and critical analysis of OCTA data may be required. The following are some tips on analysis of OCTA images.
Tip #1: Slice It and Dice It Yourself
As mentioned above, segmentation artifacts occur when the retinal layers are not identified correctly; this tends to happen when there is retinal pathology that deviates from the usual structure of the healthy adult retina. If blood vessels appear to stop abruptly and then reappear on a different OCTA layer, maintain a high degree of suspicion for errors in segmentation and manually review the segmentation of the B-scans through that area. Manually resegment the retina in at least a few B-scans through the problematic area.
Tip #2: Scroll
For pathologies in which the retinal vasculature does not conform to any retinal layers or boundaries, or pathologies in which the retinal layers have been obliterated, manually scroll through the en face OCTA data from superficial to deep. Adjust the thickness of the OCTA slab by trial and error. A prime example of this is retinoblastoma, in which the retinal architecture has been destroyed and replaced by tumor or scar.9,13
Tip #3: Understand the Definitions and Nomenclature
Different OCTA software may utilize different parameters and quantitative methods to report the OCTA findings. The cutoffs and definitions of boundaries for the superficial and deep vascular complexes are different across different software. Some software algorithms allow for the quantification of parameters such as size and circularity of the foveal avascular zone (FAZ) whereas others do not offer the capability of measuring the FAZ and require exporting images, postprocessing, and manual or self-developed software segmentation by users. Furthermore, some algorithms account for the size of the eye when measuring FAZ or other quantitative parameters, whereas others do not. Another example includes the broad term “vessel density,” which in some software is used to refer to the total area occupied by the blood vessels, and in others is used to refer to the total length density once the blood vessels have been vascularized. These differences in definition can lead to clinically important results, for example in whether or not there are significant differences in vessel density depending on race/ethnicity.14
This discrepancy across systems is also evident in the published literature surrounding OCTA findings in retinal pathologies. Some papers report the presence of a single FAZ, whereas others report one FAZ in the superficial vascular complex and another in the deep vascular complex. A recent publication by Munk et al discussed the initial stages of attempting to develop a consensus nomenclature for OCTA findings in retinal vascular diseases.15 However, they do note that further work needs to be done for images and quantifiable OCTA parameters to be comparable across systems.
Tip #4: Critical Thinking Required
As further research is done with OCTA, our understanding increases regarding various factors that may affect quantitative OCTA measurements. This should be considered when interpreting the results and comparing one OCTA image to another. For example, does positioning of the patient (supine vs upright) affect vessel density measurements? Does the same OCTA imaging software placed on a different imaging modality provide the same results? Ponugoti et al provided a preliminary exploration of these questions and noted that there were significant differences in vessel area density and vessel length density in the supine vs upright position in younger patients.16 Rommel et al investigated the effect of diurnal variations on retinal perfusion, and found that the perfusion of the superficial and deep capillary plexuses and full retina did not change significantly throughout the day.17 Further studies are under way to investigate how to best interpret OCTA measurements.
SUMMARY
An understanding of how OCTA images are created, how to image patients to avoid artifacts, and how to manually scroll through and analyze the OCTA output is key to the interpretation of OCTA images. Recognize the common artifacts: blink, motion, out of focus, media opacity, shadowing, hypertransmission, incorrect segmentation, and projection. Try obtaining and optimizing OCTA images in patients to visualize in real time how artifacts are created and avoided in real time. Manually scroll through the OCTA flow data on eyes with and without pathology rather than relying on the automatic segmentation produced by the machine. And finally, critically analyze the definitions of the OCTA measurements and how those measurements may change. RP
REFERENCES
- Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66-100. doi:10.1016/j.preteyeres.2017.07.002
- Ong SS, Hsu ST, Grewal D, et al. Appearance of pediatric choroidal neovascular membranes on optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258(1):89-98. doi:10.1007/s00417-019-04535-4
- Farecki ML, Gutfleisch M, Faatz H, et al. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-Angiography. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):913-921. doi:10.1007/s00417-017-3588-y
- Khadamy J, Abri Aghdam K, Falavarjani KG. An update on optical coherence tomography angiography in diabetic retinopathy. J Ophthalmic Vis Res. 2018;13(4):487-497. doi:10.4103/jovr.jovr_57_18
- Han IC, Tadarati M, Pacheco KD, Scott AW. Evaluation of macular vascular abnormalities identified by optical coherence tomography angiography in sickle cell disease. Am J Ophthalmol. 2017;177:90-99. doi:10.1016/j.ajo.2017.02.007
- Treister AD, Nesper PL, Fayed AE, Gill MK, Mirza RG, Fawzi AA. Prevalence of subclinical CNV and choriocapillaris nonperfusion in fellow eyes of unilateral exudative AMD on OCT angiography. Transl Vis Sci Technol. 2018;7(5):19. doi:10.1167/tvst.7.5.19
- Hsu ST, Vajzovic L. Identifying artifacts in OCT angiography. In CA Toth, SS Ong, eds: Handbook of Pediatric Retinal OCT and the Eye-Brain Connection. Elsevier; 2019:45-54.
- Hsu ST, Chen X, House RJ, Kelly MP, Toth CA, Vajzovic L. Visualizing macular microvasculature anomalies in 2 infants with treated retinopathy of prematurity. JAMA Ophthalmol. 2018;136(12):1422-1424. doi:10.1001/jamaophthalmol.2018.3926
- House RJ, Hsu ST, Thomas AS, et al. Vascular findings in a small retinoblastoma tumor using OCT angiography. Ophthalmol Retina. 2019;3(2):194-195. doi:10.1016/j.oret.2018.09.018
- Zhang M, Hwang TS, Campbell JP, et al. Projection-resolved optical coherence tomographic angiography. Biomed Opt Express. 2016;7(3):816-828. Published 2016 Feb 9. doi:10.1364/BOE.7.000816
- Patel R, Wang J, Campbell JP, et al. Classification of choroidal neovascularization using projection-resolved optical coherence tomographic angiography. Invest Ophthalmol Vis Sci. 2018;59(10):4285-4291. doi:10.1167/iovs.18-24624
- Tran-Viet D, Kelly M, Ong SS. Optimizing systems and setup for OCT and OCTA imaging of children and infants in the nursery, clinic, and operating room. In CA Toth, SS Ong, eds: Handbook of Pediatric Retinal OCT and the Eye-Brain Connection. Elsevier; 2019:6-17.
- Thomas AS, Hsu ST, House RJ, et al. Microvascular features of treated retinoblastoma tumors in children assessed using OCTA. Ophthalmic Surg Lasers Imaging Retina. 2019;51(1):43-49. doi:10.3928/23258160-20191211-06
- Hsu ST, Ngo HT, Stinnett SS, et al. Assessment of macular microvasculature in healthy eyes of infants and children using OCT angiography. Ophthalmology. 2019;126(12):1703-1711. doi:10.1016/j.ophtha.2019.06.028
- Munk MR, Kashani AH, Tadayoni R, et al. Standardization of OCT angiography nomenclature in retinal vascular diseases: first survey results. Ophthalmol Retina. 2021;5(10):981-990. doi:10.1016/j.oret.2020.12.022
- Ponugoti A, Ngo H, Kelly MP, et al. Assessment of retinal microvasculature in supine vs upright positioning using OCT-Angiography. Invest Ophthalmol Vis Sci. 2020;61(7):5338-5338.
- Rommel F, Rothe M, Kurz M, Prasuhn M, Grisanti S, Ranjbar M. Evaluating diurnal variations in retinal perfusion using optical coherence tomography angiography. Int J Retina Vitreous. 2020;6:1-6.








