Widefield imaging has enabled retina specialists to document and assess the retinal periphery, thereby revolutionizing the understanding of retinal disorders. Specifically, this imaging technique has facilitated the ability to diagnose and manage intraocular tumors. This article will highlight the role of widefield fundus photography and imaging modalities including fluorescein angiography, indocyanine green angiography, and fundus autofluorescence as it applies to ocular oncology.
DEFINITION
Traditional fundus cameras are limited to capturing only 30° to 50° of the retina. Montage images have been used to overcome the limitations of standard fundus photos. The Early Treatment Diabetic Retinopathy Study developed a protocol that captured up to 75° by creating a montage of 7 standard 30° fields.1
With the advent of widefield imaging systems, the need for standardized terminology was initially addressed by the Diabetic Retinopathy Clinical Research Network (DRCR Retina Network). They adopted the “field of view” approach as a framework and defined ultrawidefield (UWF) fundus images as those that have at least a 100° view of the fundus.2 More recently, the International Widefield Imaging Study Group developed a definition that used a different approach, utilizing anatomical landmarks. This is reflective of the process of performing clinical fundus exams, with widefield images defined as single capture images that extend up to and include vortex vein ampullae in all 4 quadrants. Within this paradigm, UWF images are those that include the retina anterior to the vortex vein ampullae in all 4 quadrants in a single capture. Finally, panretinal images span ora to ora in 360° and currently rely on montage techniques.3
IMAGING SYSTEMS
An array of options exists for choosing widefield imaging systems, such as RetCam (Natus), Clarus (Clarus 500, Zeiss Inc), Mirante (Nidek Inc), and Spectralis (Spectralis, Heidelberg Inc). The RetCam is a contact-based system that can image up to 130° of retina that is primarily used in neonatal and pediatric settings. The Clarus UWF system is noncontact and captures up to 133° while preserving the true color of the retina. Mirante is a multimodal imaging system that includes a view of 163° of the retina. The Heidelberg Spectralis wide-angle system captures 105° by using a noncontact lens while minimizing lash artifact.
The Optos (Optos Inc) UWF system relies on confocal scanning laser ophthalmoscopy (cSLO) technique. It is a noncontact device that can image up to 200° of the fundus or 82% of the retinal surface area in a single capture. The multimodal Optos system includes fundus autofluoresence, fluorescein angiography, indocyanine green angiography, and optical coherence tomography (part of Optos Monaco). The automontage feature creates a composite of 4 gaze-steered images and can capture 220° or 97% of the retina. Optos produces high-resolution images with rapid acquisition time without the need for mydriasis, therefore making it an ideal screening tool.4 Limitations of this system include pseudocolor imaging, lash artifact, and peripheral distortion.
WIDEFIELD COLOR FUNDUS PHOTOGRAPHY
When considering intraocular tumors, there are numerous applications of widefield color fundus photography (WF-CFP). The ease of obtaining high-quality images allows for detection of fundus lesions in optometric and comprehensive ophthalmology settings, which may be difficult to observe by ophthalmoscopic examination.
Once a tumor is identified, WF-CFP can be used to document the appearance, outline, surface features, and surrounding changes, both at baseline and during subsequent visits. In comparison to standard fundus photos and montage images, WF-CFP are more likely to capture all borders of the lesion (Figure 1). Ayres et al demonstrated that UWF imaging is more consistent with clinical estimates of tumor basal diameter when compared to other diagnostic modalities, including ultrasonography.5 The images and size estimates can then be used for plaque brachytherapy planning. Widefield CFP remains a powerful tool in the posttreatment period, because response to therapy and margin stability can be assessed more readily with earlier detection of recurrence.
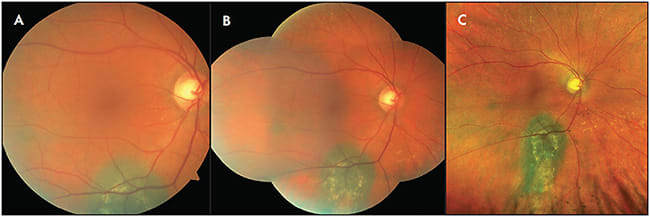
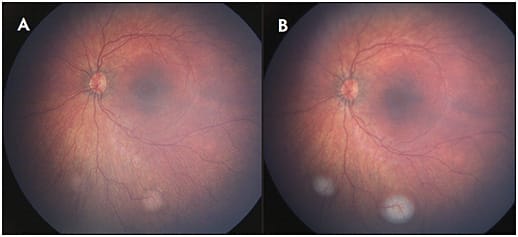
Widefield CFP can also be applied in the diagnosis and management of other tumors, such as choroidal hemangioma, metastasis, and a variety of retinal tumors. Widefield imaging techniques are particularly useful in the setting of retinoblastoma. In addition to recording the initial exam and response to therapy (Figure 2), salient features such as vitreous seeding and anterior segment involvement can be documented.6
WIDEFIELD FUNDUS FLUORESCEIN ANGIOGRAPHY
The application of widefield fundus fluorescein angiography (WF-FFA) has been integral to the thorough evaluation of intraocular tumors including retinoblastoma and peripheral vascular lesions. A common diagnostic dilemma is distinguishing between retinoblastoma and Coats disease, particularly in advanced stages where funduscopic exam is limited by vitreous and anterior-segment involvement. In this scenario, WF-FFA using Retcam remains an indispensable diagnostic tool that demonstrates characteristic findings and enables accurate diagnosis.7 Widefield FFA also plays a role during and after treatment as it can be used to demonstrate retinal and choroidal nonperfusion resulting from intra-arterial chemotherapy, intravenous chemotherapy, and intravitreal or local therapies.8
Widefield FFA has also been used to study features of retinal capillary hemangiomas (RCH), including capillary leakage, telangiectasias, and abnormal capillary networks.9 When diagnosing and treating peripheral RCH, WF-FFA aids in reliable identification of feeder vessels which are used as therapeutic targets.10 The ability to detect peripheral retinal angiomas is of great importance when considering syndromic diseases such as Von-Hippel Lindau (VHL) (Figure 3). Widefield FFA has been proposed as a screening tool in VHL clinics and is superior to WF-CFP at identifying new smaller lesions.11,12
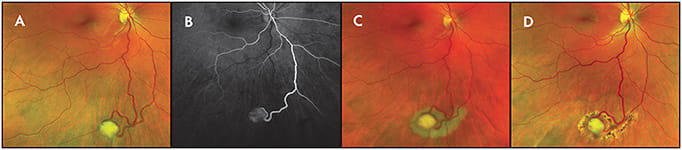
Furthermore, WF-FFA patterns are distinct for intraocular vascular tumors and can be used to delineate vasoproliferative tumors (VPT) from RCH. In contrast to RCH, VPT lack large feeder vessels and demonstrate marked hyperfluoresence and leakage due to the presence of intrinsic vessels. Widefield FFA can also be used to document and diagnose tumor-like lesions, such as exudative hemorrhagic chorioretinopathies.13,14
When examining patients with radiation retinopathy, WF-FFA is able to recognize early peripheral changes and permits accurate classification. Clinically, regions of peripheral capillary dropout and ischemia identified on WF-FFA become target areas for laser photocoagulation therapy and guide treatment of radiation retinopathy (Figure 4).6
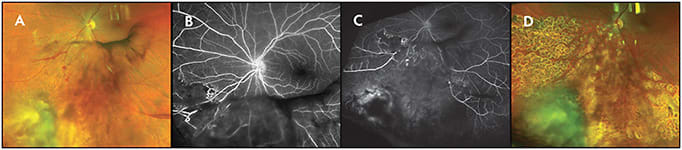
WIDEFIELD INDOCYANINE GREEN ANGIOGRAPHY
Although diagnosing choroidal tumors can be challenging, the use of widefield indocyanine green angiography (WF-ICGA) is able to differentiate between tumor types. This is particularly relevant for examining peripherally located vascular lesions, such as vortex vein varix, which is known to mimic the appearance of small choroidal melanoma. Widefield ICGA of vortex vein varix demonstrates hypercyanescence of the vortex vein ampulla with slow filling.15 This is distinct from the hypocyanescence observed with choroidal melanoma. Utility of this imaging modality is highlighted when diagnosing ill-defined choroidal lesions, including diffuse choroidal hemangioma (Figure 5) and choroidal lymphoma (Figure 6). Widefield ICGA is able to demarcate the boundaries of the tumor, which is imperative to successful treatment.

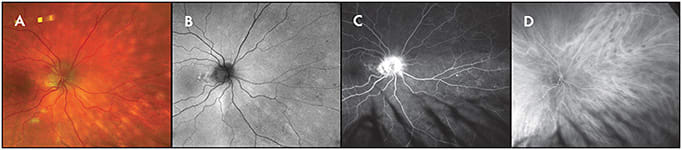
WIDEFIELD FUNDUS AUTOFLUORESCENCE
Widefield fundus autofluorescence (WF-FAF) can be used to identify features of posterior-segment tumors, including orange pigment and leakage with “guttering” appearance. In general, presence of hyperautofluorescence due to subretinal fluid indicates acute change, suggestive but not diagnostic of choroidal melanoma rather than choroidal nevus (Figure 7).16
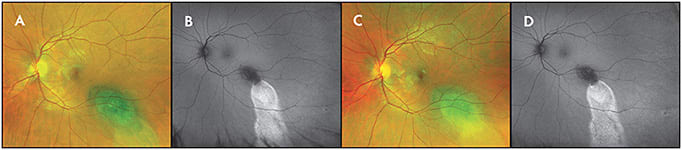
LIMITATIONS OF WIDEFIELD IMAGING IN OCULAR ONCOLOGY
Although WF imaging has become a cornerstone of ocular oncology practice, certain limitations remain. For one, there is no single-capture ora to ora imaging system, and it is possible that some peripheral lesions are being overlooked. Image distortion inherent to WF imaging systems leads to challenges in dimension estimation when assessing peripheral lesions. Specifically, horizontal axis stretching occurs, leading to the larger appearance of far nasal and temporal peripheral lesions when compared to indirect ophthalmoscopy.17 Systems such as the Optomap have created measurement algorithms to mitigate these discrepancies. The use of Optos, a cSLO system, creates “pseudocolor,” which alters the appearance of lesions and may introduce artifacts. Although current systems provide good visualization of the nasal and temporal periphery, superior and inferior views are limited.
CONCLUSIONS
The advent of widefield imaging and improvement of imaging systems over the last decade have improved documentation of intraocular tumors. It has become a mainstay in the clinical practice of ocular oncology and has numerous unique applications including screening, diagnosis, treatment planning, and monitoring of treated and untreated intraocular tumors. RP
REFERENCES
- Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1-226.
- DRCR Network. Peripheral diabetic retinopathy (DR) lesions on ultrawide-field fundus images and risk of DR worsening over time. https://public.jaeb.org/drcrnet
- Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina. 2019;3(10):843-849. doi:10.1016/j.oret.2019.05.007
- Kusumi Y, Sano M, Nakayama M, et al. [Efficacy of ultra-wide angle fundus imaging without dilated pupils in annual health check-up examination]. Nippon Ganka Gakkai Zasshi. 2016;120(1):35-40.
- Ayres B, Stacey A, Grant J, McClendon T, Demirci H. Comparative study of clinical, ultrasonographic, conventional imaging, and ultra-wide-field fundus for measurements of the longest basal diameter of choroidal tumors. Ophthalmic Surg Lasers Imaging Retina. 2017;48(6):459-464. doi:10.3928/23258160-20170601-03
- Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. Int J Retina Vitreous. 2019;5(Suppl 1):49. doi:10.1186/s40942-019-0196-5
- Kim JW, Ngai LK, Sadda S, Murakami Y, Lee DK, Murphree AL. Retcam fluorescein angiography findings in eyes with advanced retinoblastoma. Br J Ophthalmol. 2014;98(12):1666-1671. doi:10.1136/bjophthalmol-2014-305180
- Bowen RC, Raval V, Soto H, Singh AD. Intraocular tumors: angiographic patterns. Asia Pac J Ophthalmol (Phila). 2020;9(5):449-460. doi:10.1097/APO.0000000000000323
- Kumar P, Ravani R, Agarwal S, Dhanda S, Kumar V. Insights into retinal hemangioblastoma using ultra widefield imaging. Indian J Ophthalmol. 2019;67(12):2029-2034. doi:10.4103/ijo.IJO_802_19
- Chen X, Sanfilippo CJ, Nagiel A, et al. Early detection of retinal hemangioblastomas in Von Hippel-Lindau disease using ultra-widefield fluorescein angiography. Retina. 2018;38(4):748-754. doi:10.1097/IAE.0000000000001601
- Mansfield Smith S, Makam R, Sullivan L, Sandford R, Allen L. Is ultra wide-field retinal imaging alone appropriate for retinal angioma screening in lower risk subjects attending Von Hippel-Lindau (VHL) clinics?. Ophthalmic Genet. 2019;40(5):403-406. doi:10.1080/13816810.2019.1678177
- Golas L, Skondra D, Ittiara S, et al. Efficacy of retinal lesion screening in Von Hippel-Lindau patients with widefield color fundus imaging versus widefield FA. Ophthalmic Surg Lasers Imaging Retina. 2019;50(11):e260-e265. doi:10.3928/23258160-20191031-12
- Tsui I, Jain A, Shah S, Schwartz SD, McCannel TA. Ultra widefield imaging of peripheral exudative hemorrhagic chorioretinopathy. Semin Ophthalmol. 2009;24(1):25-28. doi:10.1080/08820530802520178
- Kumar V, Janakiraman D, Chandra P, Kumar A. Ultra-wide field imaging in peripheral exudative haemorrhagic chorioretinopathy (PEHCR). BMJ Case Rep. 2015;2015:bcr2015213628. Published 2015 Dec 15. doi:10.1136/bcr-2015-213628
- Reynolds MS, K.; Shields, Carol L. ICGA of vortex vein varix simulating choroidal melanoma. Retina Today. 2016;May/June 2016.
- Singh AD, Grossniklaus HE. What’s in a name? Large choroidal nevus, small choroidal melanoma, or indeterminate melanocytic tumor. Ocul Oncol Pathol. 2021;7(4):235-238. doi:10.1159/000516536
- Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis(®) noncontact ultra-widefield module versus the Optos(®) Optomap(®). Clin Ophthalmol. 2013;7:389-394. doi:10.2147/OPTH.S41731








