Photobiomodulation (PBM) therapy involves low intensity levels of light at specific wavelengths, predominantly in the visible to near-infrared (NIR) regions, that modulate biological processes at the cellular level. Photobiomodulation has evolved to treat many different health conditions, from cognitive decline to wound healing to chronic skeletal muscle injury and pain.
Photobiomodulation influences a wide variety of cellular functions, including gene expression, cellular growth and proliferation, survival, and differentiation.1 Mitochondria contain the majority of chromophores that absorb light in the red to NIR region. Cytochrome c oxidase (CcO) appears to be the primary photoacceptor and is a critical mediator of mitochondrial electron transport pathway activity (Figure 1).2 Activation of CcO triggers a series of biochemical cascades. Increased adenosine triphosphate (ATP) production, reduction of reactive oxygen species (ROS), and increased antioxidant protection via PBM all support recovery of cellular function. Evidence supports PBM regulation of gene expression and activation of transcription factors, which lead to the modulation of Ca2+ signaling and a number of pathways related to cell death, stress, and inflammation.3-5 The retina is highly energy dependent and vulnerable to mitochondrial dysfunction. The retinal neurons, photoreceptors, and ganglion cells contain the highest density of mitochondria and therefore present as potential therapeutic targets for PBM.6 Preclinical studies have demonstrated that PBM can reduce cell death and mitigate oxidative stress and retinal immune response in cell culture.7 In animal models, PBM can reduce photooxidative stress,8 hyperoxic stress,9 retinopathy of prematurity models,4 and aging10 by modulating anti-inflammatory gene and protein expressions leading to the reduction of complement pathway mediators such as C2a and C3a in the retina.10-12

Lumithera developed the first multiwavelength PBM device to treat ocular indications, the Valeda Light Delivery System. Valeda is CE-marked and commercially available in Europe and select countries in South America. Valeda is designed to emit 590 nm, 660 nm, and 850 nm wavelengths using specific light-emitting diodes (LEDs). The use of 590 nm was chosen based on the inhibition of vascular endothelial growth factor (VEGF) expression in retinal cell cultures following exposure to PBM of various wavelengths.13 The 660 nm and 850 nm wavelengths were chosen based on their direct interaction with CcO. Cytochrome c oxidase is an inner mitochondrial membrane enzyme and complex IV of the electron transport chain, and a target chromophore for both of these wavelengths.14 Near infrared (850 nm) is known to be absorbed by copper CuA moieties inside CcO important to support electron flow from cytochrome C into the CcO complex. Red (660 nm) wavelengths are known to be absorbed by copper CuB moieties inside CcO and has been demonstrated to enhance O2 binding at the active site.15 Both wavelengths have independent stimulatory effects upon CcO enzyme activity, thereby restoring mitochondrial membrane potential and leading to enhanced ATP production.
PHOTOBIOMODULATION IN AGE-RELATED MACULAR DEGENERATION
Multiple clinical studies have investigated the efficacy and safety of PBM in dry age-related macular degeneration (AMD) subjects. Using the multiwavelength approach with Valeda, clinical studies have shown statistically significant benefits following PBM treatment on visual acuity and other functional parameters as well as on disease morphology in patients with dry AMD.16,17
The LIGHTSITE I pilot study (2018) evaluated PBM effect in 30 subjects (46 eyes) randomized 1:1 (PBM:sham). Subjects had a duration of dry AMD for 7.8 years after dry AMD diagnosis. Treatment with PBM followed a set protocol of 9 treatment sessions over 3 weeks (ie, 3x/week over 3 weeks) delivered at baseline and repeated at 6 months. Subjects showed a best corrected visual acuity (BCVA) mean letter score gain of ~4 letters immediately after each PBM treatment series at month 1 and month 7. Approximately 50% of PBM-treated subjects showed improvement of ≥5 letters vs 13.6% in sham-treated subjects at month 1. Statistically significant improvements in contrast sensitivity, central drusen volume, central drusen thickness, and quality of life were observed (P<.05) (Figure 2). No device-related serious adverse events were reported. High-responding subjects (≥5 letter improvement) in the PBM-treated group showed a gain of 8 letters after initial treatment (P<.01) and exhibited earlier stages of AMD disease.17
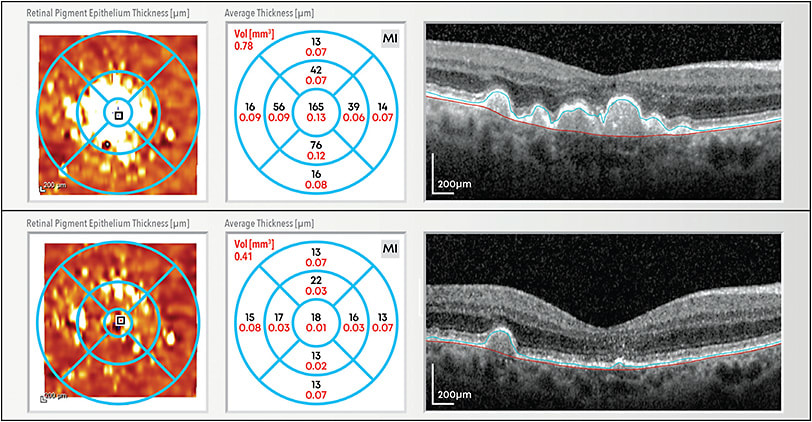
The LIGHTSITE II study (2021) was a prospective, double-masked, randomized, multicenter clinical trial conducted in 8 leading retinal centers across Europe. Intermediate dry AMD subjects received 3 rounds of PBM delivered every 4 months for a duration of 10 months. Forty-four subjects were enrolled in a 2:1 ratio of PBM:sham in the treatment groups. The mean age of the subjects was 74.1 years and mean dry AMD duration was 3.7 years after diagnosis. A statistically significant improvement in BCVA at 9 months from baseline (P=.01) in PBM-treated subjects was seen. The mean vision benefit for the PBM group vs the sham-treated group at 9 months was approximately 4 letters vs a 0.5-letter gain in the sham-treatment group.18
The ELECTROLIGHT study (2021) further explored PBM effect in dry AMD in addition to the impact on electroretinography (ERG) outcomes as a quantitative measure of functional vision improvement. Multiluminance ERG magnitude AUC improved by 14.4% from baseline after completion of the first 9 treatments and showed a 9% improvement at 6 months in the intention-to-treat population. A positive correlation between multiluminance ERG and BCVA was seen (P<.05) following initial PBM treatment. Positive correlations between multiluminance ERG and fixed luminance (R=.870) and chromatic ERG outcomes (R=.676) were also reported. Subjects showed approximately 12.8±0.98 letter improvement in BCVA at month 6 compared to baseline scores. Mars contrast sensitivity (CS) also showed improvement from baseline to month 6 at 40 cm (0.202 log±.02), 80 cm (0.197 log±.02), and 120 cm (0.28 log±.03).19
The LIGHTSITE III pivotal trial is currently ongoing in the United States with 100 intermediate dry AMD patients. A 13-month interim analysis is expected in the first quarter of 2022.
PHOTOBIOMODULATION IN RETINAL VASCULAR DISEASES
A pilot study using the Valeda device and its multiwavelength PBM approach involved 18 subjects (30 eyes; unpublished data) with good vision but signs of diabetic macular edema (DME). After treatment with PBM, 28.6% of eyes showed a resolution of intraretinal fluid and 40% of eyes showed resolution of hard exudates. Central retinal thickness remained stable, from 302±58 µm at baseline to 296±47 µm after finalizing the treatment circle of 9 treatment sessions. No signs of phototoxicity, such as disruption of the photoreceptor layers or the retinal pigment epithelium, was observed during the treatment and up to 16 months followup (unpublished data). Visual acuity remained stable at 0.1±0.1 logMAR. Most patients noted a considerable improvement in their subjective vision and reported improvement in their quality of life.20
The DRCR Retina Network evaluated in a randomized study the efficacy of a single wavelength (690 nm) in patients with center-involved DME and good vision. The study found the single wavelength approach was well tolerated using a home wearable but was not superior to placebo in reduction of central retinal thickness.21 A clinical study evaluating PBM effect in both AMD and DME/diabetic retinopathy patients using a Joovv device emitting red/infrared LED (660 nm and 810 nm) is ongoing.22 A study in central or branch retinal vein occlusion using NIR PBM is currently ongoing.23
PHOTOBIOMODULATION POTENTIAL IN OTHER OPHTHALMIC INDICATIONS
The anti-inflammatory and cell-protection mechanisms induced by PBM suggest a potential for this technology to be effective in additional ophthalmic indications, especially those with mitochondrial dysfunction contributing to disease pathology. In general, neuronal damage plays a key role in many ophthalmic pathologies, and often no effective treatment is available. Leber hereditary optic neuropathy (LHON), a rare, maternally inherited optic neuropathy caused by mitochondrial DNA point mutations, may benefit from PBM therapy and its effects on mitochondrial energy levels and function.24 In addition, many inherited retinal pathologies are associated with neurodegeneration and retinal atrophy — both processes that may be slowed using PBM.25 A recent report shows beneficial effects of PBM in Stargardt disease, a common hereditary macular degeneration disease characterized by mitochondrial dysfunction.26 Furthermore, a significant body of literature demonstrates compelling evidence of the efficacy of PBM to support wound healing, an approach that may be explored in ocular surgery and ocular injury.27
SUMMARY
Collective evidence suggests that PBM therapy has multiple effects on cellular metabolism, enhances the health of cells, and reduces degenerative and inflammatory processes at a cellular and subcellular level. Evidence from several clinical studies support the use of PBM as a potential treatment strategy in ocular disease. Larger randomized and controlled studies are ongoing to further support the clinical efficacy and safety of PBM for patients with retinal diseases. RP
REFERENCES
- Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1-17. doi: 10.1016/S1011-1344(98)00219-X
- Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11(57):154-159.
- Cardoso LM, Pansani TN, Hebling J, de Souza Costa CA, Basso FG. Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers Med Sci. 2020;35(5):1205-1212. doi: 10.1007/s10103-020-02974-8
- Natoli R. Valter K, Barbosa M, et al. 670nm photobiomodulation as a novel protection against retinopathy of prematurity: evidence from oxygen-induced retinopathy models. PloS One. 2013;8(8):e72135. doi: 10.1371/journal.pone.0072135
- Benson P, Kim JY, Riveros C, Camp A, Johnstone DM. Elucidating the time course of the transcriptomic response to photobiomodulation through gene co-expression analysis. J Photochem Photobiol B. 2020;208:111916. doi: 10.1016/j.jphotobiol.2020.111916
- Lock JH, Irani NK, Newman NJ, Neuro-ophthalmic manifestations of mitochondrial disorders and their management. Taiwan J Ophthalmol. 2021;11(1):39-52. doi: 10.4103/tjo.tjo_68_20
- Lu YZ, Natoli R, Madigan M, et al. Photobiomodulation with 670 nm light ameliorates Müller cell-mediated activation of microglia and macrophages in retinal degeneration. Exp Eye Res. 2017;165:78-89. doi: 10.1016/j.exer.2017.09.002
- Albarracin R, Eells J, Valter K, Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3582-3592. doi: 10.1167/iovs.10-6664
- Albarracin R, Natoli R, Rutar M, Valter K, Provis J. 670 nm light mitigates oxygen-induced degeneration in C57BL/6J mouse retina. BMC Neurosci. 2013;14:125. doi: 10.1186/1471-2202-14-125
- Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PloS One. 2013;8(2):e57828. doi: 10.1371/journal.pone.0057828
- Saliba A, Du Y, Liu H, et al. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: evidence from intervention studies in pigmented mice. PloS One. 2015;10(10):e0139003. doi: 10.1371/journal.pone.0139003
- Rutar M, Natoli R, Albarracin R, Valter K, Provis J. 670-nm light treatment reduces complement propagation following retinal degeneration. J Neuroinflammation. 2012;9:257. doi: 10.1186/1742-2094-9-257
- Szymanska J, Goralczyk K, Klawe JJ, et al. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J Physiol Pharmacol. 2013;64(3):387-391.
- Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761-4771. doi: 10.1074/jbc.M409650200
- Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28(2):159-160. doi: 10.1089/pho.2010.2789
- Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017;95(4):e270-e277. doi: 10.1111/aos.13354
- Markowitz SN, Devenyi RG, Munk MR, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40(8);1471-1482. doi: 10.1097/IAE.0000000000002632
- Burton B, et al. LIGHTSITE II: a double-masked, randomized, sham-controlled, parallel group, multi-center study to assess the safety and efficacy of photobiomodulation (PBM) in subjects with dry age-related macular degeneration (AMD). Poster presented at: EURETINA; September 9-12, 2021; virtual meeting.
- Perich L, et al. Improvement of visual function and electroretinography following photobiomodulation (Valeda light delivery system) treatment in dry age-related macular degeneration subjects (ELECTROLIGHT) (final analysis). Poster presented at: EURETINA; September 9-12, 2021; virtual meeting.
- Inken S, Schwahn H, Munk MR, et al., Non-invasive treatment of early diabetic macular edema by multi-wavelength photobiomodulation with the Valeda light delivery system. Invest Ophthal Vis Sci. 2021;62(8):1066.
- Kim JE, Glassman AR, Josic K, et al; DRCR Retina Network. A randomized trial of photobiomodulation therapy for center-involved diabetic macular edema with good visual acuity (Protocol AE). Ophthalmol Retina. 2021;S2468-6530(21)00312-2. doi: 10.1016/j.oret.2021.10.003
- Photobiomodulation & ketogenic diet for treatment of mid-periphery retinal disorders for Alzheimer’s disease prevention. ClinicalTrials.gov identifier: NCT03859245. Updated March 4, 2019. Accessed March 4, 2022. https://clinicaltrials.gov/ct2/show/NCT03859245
- Near-infrared light photobiomodulation treatment for retinal vein occlusion macular oedema (NIRVO). ClinicalTrials.gov identifier: NCT04847869. Updated April 19, 2021. Accessed March 4, 2022. https://clinicaltrials.gov/ct2/show/NCT04847869
- Leruez S, Amati-Bonneau P, Verny C, et al. Mitochondrial dysfunction affecting visual pathways. Rev Neurol (Paris). 2014;170(5):344-354. doi: 10.1016/j.neurol.2014.03.009
- Bagli E, Zikou AK, Agnantis N, Kitsos G. Mitochondrial membrane dynamics and inherited optic neuropathies. In Vivo. 2017;31(4):511-525. doi: 10.21873/invivo.11090
- Scalinci SZ, Valsecchi N, Pacella E, Trovato Battagliola E. Effects of photo-biomodulation in Stargardt disease. Clin Ophthalmol. 2022;16:85-91. doi:10.2147/OPTH.S344378
- Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regen Med. 2016;11(1):107-122. doi: 10.2217/rme.15.82








