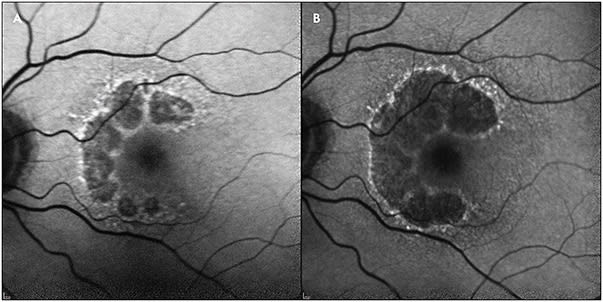
The arrival of anti-VEGF agents to treat neovascular age-related macular degeneration (AMD) has been transformative for the treatment and prognosis of this common, frequently blinding disorder. Development of a similarly transformative treatment for dry AMD has been the focus of tremendous investigation in recent years. Much of this research has focused on inhibition of the complement cascade, with more than a dozen promising treatments currently in development targeting this pathway (Table 1). The most common endpoint in these studies has been to reduce the progression of geographic atrophy (GA), the most advanced and visually debilitating form of dry AMD, as measured by fundus autofluorescence (FAF) (eg, the CHROMA, SPECTRI, DERBY, OAKS, GATHER1, and GATHER2 studies) (Figure 1).
| DRUG NAME (MANUFACTURER) | DESCRIPTION | STATUS |
| Pegcetacoplan (APL-2; Apellis Pharmaceuticals Inc.) | C3 inhibitor | Phase 3 DERBY/OAKS trial results released Q3 2021, NDA submission to FDA expected first half 2022. |
| Avacincaptad pegol (Zimura; Iveric Bio) | C5 inhibitor | Phase 2/3 GATHER1 trial complete. Phase 3 GATHER2 ongoing, top-line results expected second half 2022. |
| GEM103 (Gemini Therapeutics) | Recombinant CFH | Phase 2a ReGAtta trial ongoing, initial results demonstrated safety did not slow GA progression. |
| IONIS-FB-LRx (Ionis Pharmaceuticals) | Anti-sense CFB inhibitor | Phase 2 GOLDEN trial ongoing, expected results second half 2022. |
| GT005 (Gyroscope Therapeutics) | Subretinal AAV2 CFI | Phase 1/2 FOCUS trial interrim results announced Q1 2021, Phase 2 EXPLORE and HORIZON trial top-line data expected 2023. Acquisition of Gyroscope by Novartis announced Q4 2021. |
| NGM621 (NGM Biopharmaceuticals) | anti-C3 monoclonal antibody | Phase 1 study well tolerated. Phase 2 CATALINA trial ongoing, completion expected 2023. |
| ANX007 (Annexon Biosciences) | Anti-C1q antibody | Phase 1b studies demonstrated favorable safety profile and C1q inhibition for up to 2 months. Phase 2 ARCHER trial ongoing, results expected 2023. |
| Danicopan (ALXN2040; Alexion) | Complement FD inhibitor | Phase 2 trial ongoing. |
| HMR59 (AAVCAGsCD59) (Hemera Biosciences, Janssen Pharmaceuticals) | Intravitreal AAV2 sCD59 | Phase 2 trial is planned. |
| RO7303359 (Hoffman La Roche) | Unclear | Phase 1 estimated complete February 2022. |
| BI 754132 (Boehringer Ingelheim) | Unclear | Phase 1 estimated complete March 2022. |
| ONL1204 (ONL Therapeutics) | Fatty acid synthase inhibitor | Phase 1 trial estimated complete September 2022. |
| ALXN1720 (Alexion) | anti-C5 antibody | Phase 1 trial delayed due to COVID-19 pandemic. |
| CB2782-PEG (Catalyst Biosciences/Biogen) | C3 degrader | Preclinical development.Acquisition of Gyroscope by Novartis announced Q4 2021. |
| GT011 (Gyroscope Therapeutics) | CFH like-1 gene therapy | Preclinical development. Acquisition of Gyroscope by Novartis announced Q4 2021. |
A variety of agents are also in development that employ non-complement-mediated approaches to treat dry AMD and GA. In earlier stages of development, these emerging approaches also merit investigation to address the complex and multifactorial pathophysiology of a disease like AMD. While the development pathway in dry AMD and GA has been littered by many failed trials, from each of these, we have continued to expand our understanding of this complex and multifactorial disease and how best to study it.
ANTI-INFLAMMATORY AGENTS
Inflammatory mediators are implicated in AMD pathogenesis, where they contribute to choriocapillaris endothelial cell damage and breakdown in Bruch’s membrane. Targeting these pathways may rescue endothelial cells, reduce deposition of inflammatory components that promote drusen formation, and prevent retinal pigment epithelium (RPE) atrophy.1
Doxycycline is a commonly used tetracycline antibiotic that also possesses multiple anti-inflammatory properties, including inhibition of matrix metalloproteinases, scavenging of reactive oxygen species (ROS), inhibition of leukocyte chemotaxis, and reduction of inflammatory cytokine signaling.2 Oracea (Galderma Laboratories), a daily 40-mg oral doxycycline formulation, is currently under investigation in a randomized, double-blind, placebo-controlled, phase 3 clinical trial. Results are pending.3 Similarly, a smaller phase 2 clinical trial of oral minocycline for reducing the rate of GA progression is currently under way, with results expected in late 2022.4
High temperature requirement factor A1 (HTRA1) serine protease inhibition is promising as a therapeutic target given its significance in genome-wide association studies and its suspected role in degradation of the Bruch’s membrane extracellular matrix.1,5 Homozygosity with high-risk HTRA1 haplotypes is also associated with increased serum C-reactive protein.6 FHTR2163 (Genentech) is an antigen-binding fragment (Fab) of a monoclonal antibody targeting HTRA1. Results from an open-label, multicenter phase 1 study were recently published assessing intravitreal injection of FHTR2163.5 No significant adverse events or dose-limiting toxicities were reported, and sustained reduction in the HTRA1-specific substrate DKK3 was observed. A phase 2 clinical trial assessing the utility of FHTR2163 in reducing progression of GA is in recruitment.7
The NLRP3 inflammasome is a key regulator of proinflammatory signaling and has been implicated in AMD pathogenesis. NLRP3 activation by β-amyloid oligomers within the RPE leads to caspase activation and production of proinflammatory IL-1β and IL-18.8 Kamuvudines, modified nucleoside reverse-transcriptase inhibitors, inhibit NLRP3 without antiretroviral activity or the side effects associated with traditional nucleoside reverse-transcriptase inhibitors. Kamuvudines prevent inflammasome activation following cytosolic escape of mitochondrial DNA and preventing inflammasome-mediated RPE degeneration.9 Several kamuvudines are in preclinical development by Inflammasome Therapeutics as potential therapies for prevention of GA with clinical trials expected to begin in 2022.10
Xiflam (Ocunexus Therapeutics/Inflammx) is an oral small-molecule inhibitor of the Connexin-43 hemichannel that aims to reduce release of proinflammatory mediators, as well as NLRP3 inflammasome activation. Phase 2b clinical trials are in planning.11
VISUAL CYCLE MODULATION
Other agents aim to modulate the visual cycle to reduce deposition of toxic visual cycle byproducts. In particular, the vitamin A derivative A2E is toxic to the RPE and may hasten progression of GA. Unfortunately, efforts to reduce RPE toxicity by inhibiting formation of A2E have met with limited success to date. Emixustat (Acucela Inc), an oral small-molecule RPE65 inhibitor, did not reduce the growth rate of GA in phase 2b/3 clinical trials.12 Fenretinide (Revision Therapeutics), an oral inhibitor of retinol-binding protein, also failed to reduce GA growth rate compared to placebo in phase 2 trials.13
However, promising visual cycle modulators may be on the horizon. ALK-001 (Alkeus Pharmaceuticals), an oral C20-D3 deuterated vitamin A, is designed to reduce formation of A2E production and is currently in phase 3 studies with results expected in 2022.14 ALK-001 was also recently granted Breakthrough Therapy Designation by the FDA for the treatment of Stargardt disease.15
NEUROPROTECTION AND MITOCHONDRIAL ENHANCEMENT
Neuroprotection has also been investigated as a strategy for the rate of GA progression by reducing neuroretinal cell death. This approach is particularly compelling given the multifactorial pathogenesis of GA and the numerous stressors that neuroretinal tissues face in this disease context, including a variety of oxidative, inflammatory, ischemic, and metabolic factors.
Brimo DDS (Allergan) is an intravitreal biodegradable polymer implant containing brimonidine, an alpha-2 adrenergic agonist that has been shown to ameliorate phototoxic damage in primate models.16 The implant contains a delayed delivery system (DDS) designed to release the drug over a 6-month period. In preclinical study, brimonidine enhanced the ability of RPE and photoreceptors to resist oxidative and phototoxic damage. A phase 2a trial of Brimo DDS containing either 132 μg or 264 μg brimonidine demonstrated a significant reduction in GA growth rate at month 3 and a favorable safety profile with both doses; most adverse affects were related to intravitreal injection.17 A larger phase 2b trial (BEACON) evaluated a Brimo DDS implant containing 400 µg brimonidine and demonstrated a modest reduction of 10% and 12% in GA progression at 24 months and 30 months.18 The BEACON trial was halted at interim analysis due to a slow GA progression rate (approximately 1.6 mm2/year) in the enrolled subjects. Two phase 3 trials evaluating Brimo DDS at 200 μg and 400 μg are in planning.
Risuteganib (Allegro Ophthalmics) is a novel small peptide integrin inhibitor that is administered by intravitreal injection.19 Preclinical studies demonstrated that risuteganib reduced RPE cell damage from oxidative stress and reduced expression of cytotoxic gene transcripts, including BAX and VEGFA. Results of a phase 2a, prospective, double-masked, sham-controlled trial were recently published and demonstrated that the proportion of subjects meeting the primary endpoint of an 8 ETDRS letter or more gain in best-corrected visual acuity (BCVA) at week 28 vs baseline was 48% in the risuteganib group compared to 7% in the sham group (P=.013). Twenty percent of patients in the risuteganib group gained 15 letters or more, while none in the sham arm reached this degree of visual acuity gain. No significant adverse effects were observed.20 A larger phase 2b/3 trial is in planning. AffaMed Therapeutics has recently entered into a $145 million licensing agreement with Hanmi Pharmaceutical to develop and commercialize risuteganib for the treatment of intermediate dry AMD in China, Hong Kong, Taiwan, and Macau.21
Elamipretide (Stealth Biotherapeutics Inc) is a novel agent that binds cardiolipin within mitochondrial cristae. In GA, ROS causes loss of cristae curvature and disrupts respiratory complex proteins.22 Elamipretide stabilizes cristae architecture, thereby promoting mitochondrial respiration and reducing formation of ROS and mitochondrial loss. Elamipretide was well tolerated in phase 1 trials, which assessed safety and tolerability of elamipretide both for noncentral GA and for high-risk drusen. Post-hoc analysis of the phase 1 Re-CLAIM trial demonstrated that improvements in low-luminance BCVA in patients treated with elamipretide were correlated with OCT parameters, including reduction of macular ellipsoid zone (EZ) attenuation and preservation of mean macular retinal thickness and macular EZ-RPE volume.23 A multicenter, placebo-controlled phase 2 trial (Re-CLAIM-2) evaluating the effect of daily subcutaneous elamipretide on low-luminance BCVA and rate of GA progression is ongoing, with results expected in the second quarter of 2022.24
REDUCTION OF TOXIC BYPRODUCTS
Several agents aim to reduce formation of toxic byproducts that contribute to drusen formation. β-amyloid oligomers have been identified as a component of drusen and accumulate in the outer retina, RPE, and choriocapillaris in aging retinal tissues, where they may serve as a scaffold for deposition of other drusen components and activate complement.25 While several recent drug candidates in this space failed to show efficacy in phase 2 clinical trials, such as GSK933776 (GlaxoSmithKline), other promising agents are in development.26
GAL-101 (Galimedix Therapeutics) is an amyloid-β (Aβ) aggregation inhibitor. GAL-101 binds to Aβ monomers and inhibits assembly of toxic Aβ oligomers, instead allowing Aβ monomers to assemble into nontoxic clusters. Preclinical data in a murine RPE model demonstrated a reduction in amyloid and C3 staining of the photoreceptor layer, RPE, and Bruch’s membrane.27,28 Topical GAL-101 was well tolerated with minimal systemic distribution in a phase 1 study. A phase 2 study is in planning.
ALZ-507 (Alzheon) is an amyloid oligomerization inhibitor that is currently in preclinical development. Alzheon is planning for FDA IND filing in 2022.29
RT011 (Retrotope) is an orally administered, isotopically stabilized docosahexaenoic acid (DHA), a polyunsaturated fatty acid that is designed to reduce lipid peroxidation. By reducing DHA lipid peroxidation, RT011 aims to reduce the buildup of toxic lipid byproducts that contribute to drusen formation and aid neuroretinal cells in resisting oxidative stress. Retrotope announced positive results from preclinical studies at the second annual Dry AMD Therapeutic Development Summit in October 2021. Several presentations at the summit reported that RT011 delivered dose-dependent protection against oxidative damage and reduced retinal cellular damage in an animal model. A 26-week oral toxicity study was also recently completed and found RT011 to be well tolerated at all doses tested. Retrotope plans for FDA IND filing in the first half of 2022 and for initiating clinical studies in 2022.30
AUTOPHAGY
Promotion of RPE autophagy as a strategy to reduce accumulation of lipid-based drusenoid deposits has thus far been met with mixed success. Attempts to induce autophagy via inhibition of mTOR using intravitreal sirolimus did not reduce GA growth rate and resulted in several cases of sterile endophthalmitis in a randomized phase 2 trial, resulting in the trial’s early termination.31 However, investigations of the FDA-approved anti-helminthic agent flubendazole have shown that this agent may hold promise as a treatment for dry AMD. A recent preclinical study demonstrated that flubendazole reduced apolipoprotein secretion, increased production of the lipid degradation byproduct β-hydroxybutyrate, and decreased lipofuscin-induced cellular and tight-junction disruption in human fetal RPE culture via an mTOR-independent mechanism. Flubendazole also caused toxic lipofuscin-like granules to compact into a possibly less toxic form.32
STEM CELL THERAPY
Several cell-based therapies are in development for treatment of dry AMD. These treatments aim to deliver stem cell–derived RPE cells into the subretinal space to replace or regenerate the RPE cells that are lost or damaged in GA. Importantly, these stem cell studies are all FDA registered and compliant, and do not charge patients for participation. Unregulated clinics have blinded several patients when they have isolated adipose tissue-derived “stem cells” and injected them intravitreally for the treatment of dry AMD.33
Opregen (Lineage Cell Therapeutics/Roche/Genentech) is an allogeneic RPE cell transplant that is delivered by subretinal injection. Opregen is currently in phase 1/2a clinical trials evaluating a single subretinal injection in patients with dry AMD with GA. Enrollment was completed in November 2020. Interim results of the study were presented at the Retina Society’s 2021 annual meeting.34 In the interim analysis, Opregen was well tolerated, with most adverse effects being related to pars plana vitrectomy or use of the Orbit Subretinal Delivery System.
In November 2021, Lineage announced that 4 patients treated with Opregen demonstrated retinal tissue restoration with new areas of RPE with overlying ellipsoid zone, external limiting membrane, and outer nuclear layers, as well as reduced GA area. These findings may suggest that Opregen is capable of not only slowing but also reversing retinal damage in GA. Engagements with the FDA to plan later-stage clinical trials are planned for the first quarter of 2022.35 On December 20, 2021, Lineage announced a global licensing agreement with Genentech/Roche for the development and commercialization of Opregen. Under the agreement, Lineage will complete current phase 1/2a clinical studies and perform certain manufacturing roles, while Genentech will pursue further clinical development of Opregen.36
CPCB-RPE1 (Regerative Patch Technologies) is an ultrathin (1-6 µm) subretinal implant composed of a synthetic parylene membrane containing a polarized monolayer of human embryonic stem cell–derived RPE that is currently in phase 1/2a trials. One-year results of the phase 1/2a trial were recently published.37 Sixteen patients with dry AMD with GA and BCVA ≤20/200 were treated. The implant was well tolerated, with most adverse effects related to the implant delivery. While the study was not powered for efficacy, 27% of patients demonstrated a greater than 5 letter gain in BCVA compared to the untreated eye, while a greater proportion of untreated eyes demonstrated BCVA loss. This difference was not statistically significant.
ASP7317 (Astellas) is an investigational therapy involving subretinal injection of stem cell–derived RPE cells. ASP7317 is a new cell line that follows Astellas’ previous MA09-hRPE cell line, which was well tolerated in phase 1/2 studies of patients with dry AMD and Stargardt disease.38 A phase 1 trial assessing the safety and tolerability of ASP7317 is under way, although screening and enrollment are paused due to COVID-19 and manufacturing delays. The study is estimated to complete Q4 2022.39
CNTO-2476 (Janssen) is an investigational cell-based therapy involving subretinal delivery of human umbilical cord tissue–derived cells, which demonstrated secretion of neurotrophic factors in animal models, suggesting a possible role in providing trophic support to existing RPE cells and photoreceptors. Results of the phase 2b PRELUDE study evaluating a suprachoroidal delivery system recently demonstrated successful delivery with no significant adverse effects, although no improvement in visual acuity or reduction in GA size or growth rate was observed.40
SUMMARY
The year 2022 and beyond promise to be exciting for dry AMD therapies. Numerous important developments are on the horizon for dry AMD treatment. RP
REFERENCES
- Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1-29. doi:10.1016/j.preteyeres.2014.11.005
- Henehan M, Montuno M, De Benedetto A. Doxycycline as an anti-inflammatory agent: updates in dermatology. J Eur Acad Dermatol Venereol. 2017;31(11):1800-1808. doi:10.1111/jdv.14345
- Clinical study to evaluate treatment with ORACEA for geographic atrophy (TOGA). ClinicalTrials.gov identifier: NCT01782989. Updated April 29, 2021. Accessed January 3, 2022. clinicaltrials.gov/ct2/show/NCT01782989
- Evaluation of oral minocycline in the treatment of geographic atrophy associated with age-related macular degeneration. Clinicaltrials.gov identifier: NCT02564978. Updated January 24, 2022. Accessed January 25, 2022. https://clinicaltrials.gov/ct2/show/NCT02564978
- Khanani AM, Hershberger VS, Pieramici DJ, et al. Phase 1 study of the anti-HtrA1 antibody-binding fragment FHTR2163 in geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2021;232:49-57. doi:10.1016/j.ajo.2021.06.017
- Yasuma TR, Nakamura M, Nishiguchi KM, et al. Elevated C-reactive protein levels and ARMS2/HTRA1 gene variants in subjects without age-related macular degeneration. Mol Vis. 2010;16:2923-2930.
- A study assessing the safety, tolerability, and efficacy of FHTR2163 in participants with geographic atrophy secondary to age-related macular degeneration (AMD) (GALLEGO). ClinicalTrials.gov identifier: NCT03972709. Updated December 27, 2021. Accessed January 3, 2022. clinicaltrials.gov/ct2/show/NCT0392709
- Narendran S, Pereira F, Yerramothu P, et al. Nucleoside reverse transcriptase inhibitors and Kamuvudines inhibit amyloid-β induced retinal pigmented epithelium degeneration. Signal Transduct Target Ther. 2021;6(1):149. doi:10.1038/s41392-021-00537-z
- Fukuda S, Narendran S, Varshney A, et al. Alu complementary DNA is enriched in atrophic macular degeneration and triggers retinal pigmented epithelium toxicity via cytosolic innate immunity. Sci Adv. 2021;7(40):eabj3658. doi:10.1126/sciadv.abj3658
- Inflammasome Therapeutics. Inflammasome Therapeutics’ kamuvudines continue to show promise for future treatment – and potentially a cure – for blindness in seniors. News release. September 30, 2021. Accessed January 3, 2022. https://www.inflam.com/images/pdfs-doc/2021_09_30_Inflammasome_Press_Release_Science_Advances.pdf
- InflammX Therapeutics. Ophthalmic pipeline. Accessed January 4, 2022. https://www.inflammx.com/ophthalmic
- Rosenfeld PJ, Dugel PU, Holz FG, et al. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial [published correction appears in Ophthalmology. 2019;126(3):471-472]. Ophthalmology. 2018;125(10):1556-1567. doi:10.1016/j.ophtha.2018.03.059
- Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33(3):498-507. doi:10.1097/IAE.0b013e318265801d
- Phase 3 Study of ALK-001 in Geographic Atrophy (SAGA). ClinicalTrials.gov identifier: NCT03845582. Updated July 20, 2021. Accessed January 4, 2022. https://clinicaltrials.gov/ct2/show/NCT03845582
- Alkeus Pharmaceuticals. FDA grants Alkeus Pharmaceuticals breakthrough therapy designation for ALK-001 (C20-D3-vitamin A) for the treatment of Stargardt disease. News release. July 14, 2021. Accessed January 4, 2022. https://www.alkeuspharma.com/2021-07-14-press-release.html
- Rajagopalan L, Ghosn C, Tamhane M, et al. A nonhuman primate model of blue light-induced progressive outer retina degeneration showing brimonidine drug delivery system-mediated cyto- and neuroprotection. Exp Eye Res. 2021;209:108678. doi:10.1016/j.exer.2021.108678
- Safety and efficacy of brimonidine intravitreal implant in patients with geographic atrophy due to age-related macular degeneration (AMD). ClinicalTrials.gov identifier: NCT00658619. Updated August 21, 2018. Accessed March 10, 2022. https://clinicaltrials.gov/ct2/show/NCT00658619
- A safety and efficacy study of brimonidine intravitreal implant in geographic atrophy secondary to age-related macular degeneration (BEACON). ClinicalTrials.gov identifier: NCT02087085. Updated April 23, 2019. Accessed January 4, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02087085
- Shaw LT, Mackin A, Shah R, et al. Risuteganib-a novel integrin inhibitor for the treatment of non-exudative (dry) age-related macular degeneration and diabetic macular edema. Expert Opin Investig Drugs. 2020;29(6):547-554. doi:10.1080/13543784.2020.1763953
- Boyer DS, Gonzalez VH, Kunimoto DY, et al. Safety and efficacy of intravitreal risuteganib for non-exudative AMD: a multicenter, phase 2a, randomized, clinical trial. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):327-335. doi:10.3928/23258160-20210528-05
- AffaMed Therapeutics. AffaMed Therapeutics enters into a licensing agreement with Hanmi Pharmaceutical to develop and commercialize risuteganib (Luminate) as a first-in-class treatment for intermediate dry AMD patients in Greater China. News release. January 3, 2022. Accessed January 4, 2022. https://www.affamed.com/press-releases-30?
- Heier JS. Subcutaneous elamipretide for treatment of noncentral geographic atrophy secondary to AMD: ongoing findings from the ReCLAIM program [abstract]. Angiogenesis, Exudation, and Degeneration 2021 – Virtual Edition; February 13-14, 2021; Miami, FL.
- Pieramici D. Low-luminance visual acuity and the association of ellipsoid zone integrity in dry age-related macular degeneration patients treated with subcutaeous elamipretide: post-hoc analysis of the phase 1 ReCLAIM-1 study [abstract]. The Retina Society Annual Meeting 2021; September 30, 2021; Chicago, IL.
- ReCLAIM-2 study to evaluate safety, efficacy & pharmacokinetics of elamipretide in subjects with AMD with non-central GA (ReCLAIM-2). Clinicaltrials.gov identifier: NCT03891875. Updated November 9, 2021. Accessed January 4, 2022.https://clinicaltrials.gov/ct2/show/NCT03891875
- Lynn SA, Keeling E, Munday R, et al. The complexities underlying age-related macular degeneration: could amyloid beta play an important role?. Neural Regen Res. 2017;12(4):538-548. doi:10.4103/1673-5374.205083
- Rosenfeld PJ, Berger B, Reichel E, et al. A randomized phase 2 study of an anti-amyloid ß monoclonal antibody in geographic atrophy secondary to age-related macular degeneration. Ophthalmol Retina. 2018;2(10):1028-1040. doi:10.1016/j.oret.2018.03.001
- Galimedix Therapeutics. Clinical trials. Accessed January 4, 2022. https://www.galimedix.com/clinical-trials
- Galimedix Therapeutics. AMD preclinical data. Accessed January 4, 2022. https://www.galimedix.com/technology/amd-preclinical-data .
- Alzheon. Pipeline. Accessed January 4, 2022. https://alzheon.com/pipeline/
- Retrotope. Positive results from studies of Retrotope’s RT011 in animal models of retinal degeneration to be featured in oral presentations at 2nd annual Dry AMD Therapeutic Development Summit. News release. October 19, 2021. Accessed January 4, 2022. https://www.retrotope.com/press-release/positive-results-from-studies-of-retrotopes-rt011-in-animal-models-of-retinal-degeneration-to-be-featured-in-oral-presentations-at-2nd-annual-dry-amd-therapeutic-development-summit/
- Gensler G, Clemons TE, Domalpally A, et al. Treatment of geographic atrophy with intravitreal sirolimus: the Age-Related Eye Disease Study 2 ancillary study. Ophthalmol Retina. 2018;2(5):441-450. doi:10.1016/j.oret.2017.08.015
- Zhang Q, Presswalla F, Ali RR, Zacks DN, Thompson DA, Miller JML. Pharmacologic activation of autophagy without direct mTOR inhibition as a therapeutic strategy for treating dry macular degeneration. Aging (Albany NY). 2021;13(8):10866-10890. doi:10.18632/aging.202974
- Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. 2017;376(11):1047-1053. doi:10.1056/NEJMoa1609583
- Riemann, C. Phase 1/2a clinical trial of transplanted allogeneic retinal pigmented epithelium (RPE, OpRegen) cells in advanced dry age-related macular degeneration (AMD): interim result [abstract]. The Retina Society Annual Meeting 2021; September 30, 2021; Chicago, IL.
- Lineage Cell Therapeutics. Lineage reports fourth case of retinal tissue restoration with OpRegen. News release. November 30, 2021. Accessed January 4, 2022. https://investor.lineagecell.com/news-releases/news-release-details/lineage-reports-fourth-case-retinal-tissue-restoration-opregenr
- Lineage Cell Therapeutics. Lineage establishes exclusive worldwide collaboration with Genentech for the development and commercialization of OpRegen RPE cell therapy for the treatment of ocular disorders. News release. December 20, 2021. Accessed January 4, 2022. https://investor.lineagecell.com/news-releases/news-release-details/lineage-establishes-exclusive-worldwide-collaboration-genentech
- Kashani AH, Lebkowski JS, Rahhal FM, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10(10):13. doi:10.1167/tvst.10.10.13
- Astellas. Initiatives in cell therapy – turning innovative science into value for patients. Astellas Research and Development Meeting. December 13, 2018. Accessed January 4, 2022. https://www.astellas.com/system/files/2020-03/final_2_181212_rd_day_eg.pdf
- A study of the safety and tolerability of ASP7317 in adults who are losing their clear, sharp central vision due to geographic atrophy secondary to dry age-related macular degeneration. ClinicalTrials.gov identifier: NCT03178149. Updated November 11, 2021. Accessed January 4, 2022. https://clinicaltrials.gov/ct2/show/03178149
- Heier JS, Ho AC, Samuel MA, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration: phase 2b study. Ophthalmol Retina. 2020;4(4):384-393. doi:10.1016/j.oret.2019.11.011








