Retina specialists are increasingly seeing patients in the operating room who require complex procedures. Retinal Physician convened a roundtable of surgeons experienced in complex surgical cases to present and discuss approaches to a series of difficult cases.
Dr. Murray: Something I have been focused on is coupling anterior-segment and posterior-segment surgery when the anterior-segment pathology impedes the final visual acuity. This patient is as 76-year-old female who presented with visual acuity of 20/60. She was scheduled for cataract surgery by an outside surgeon, and in the process of a comprehensive exam, they noted a large pigmented temporal mass with an exudative retinal detachment on the margin (Figure 1; surgical video available below). That’s what prompted our evaluation. This looks like a malignant melanoma. We want, if possible, to recover best visual function for this patient and also to stabilize the tumor, ablate the tumor utilizing a laser technique, and biopsy the tumor for molecular genomics.

This is a case of a standard phaco chop, and I used a biopsy technique. We used a valved 23-gauge trocared cannula system. Because we operated on the back of the eye, I sutured the anterior segment for fluidic stability, and I think this is very important (Figure 2).

Recently, we’ve appreciated that using indocyanine green (ICG) for internal limiting membrane (ILM)-guided peeling is extremely important to prevent the development of epiretinal membrane that may affect the central vision. So, after ICG staining, we use an Alcon Finesse Flex Loop and engage the ILM, and then peel (Figure 3). The technique I prefer is to peel across the fovea as opposed to circumferentially. Small blot hemorrhages are not at all uncommon (Figure 4). We want to release any degree of tractional alterations. It’s critical to elevate the hyaloid before peeling the ILM. We’ve also shown that suppressing postoperative inflammation in these complex cases with the use of intravitreal triamcinolone acetonide is important (Figure 5).1
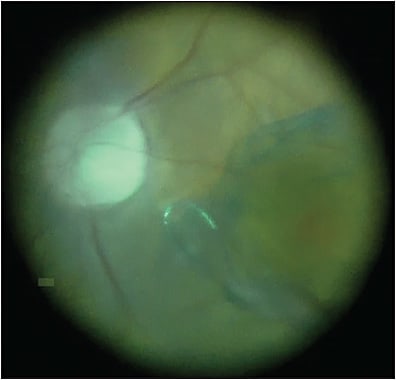
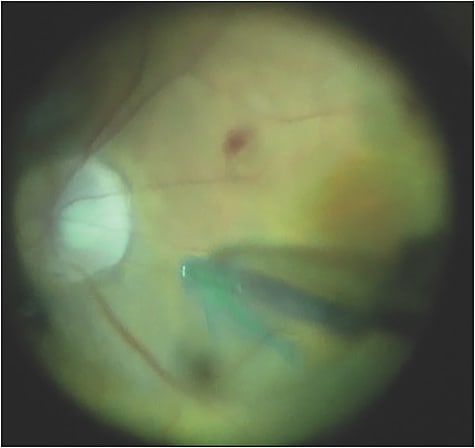

In this case, postoperative vision at 6 weeks was 20/25 uncorrected, and the patient’s tumor showed gene expression profiling molecular classification of a class 1B melanoma. In those patients treated in this manner, we do not recommend any further treatment and continue to closely observe. The complexity of this case is anterior–posterior combined surgery for a peripheral ciliochoroidal malignant melanoma and the importance of the surgical approach to include biopsy, ablation, and molecular genomics.
Dr. Kaiser: Dr. Murray, for this case, did you perform the biopsy transretinally or transsclerally?
Dr. Murray: If I’m doing plaque brachytherapy for an anterior-segment tumor and I’m not violating the globe itself, I do transscleral biopsy. However, in this case, I was doing a vitrectomy, removing the hyaloid, elevating and freeing the tractional alterations of the tumor, and sealing the tumor, so I approached transretinally. The transretinal biopsy is in the center of the tumor, surrounded by laser ablation, and we have not had any of our patients go on to detach in this setting. Overall, the report of retinal detachment is less than 1% and we now have 5-year follow-up that’s been published on this patient series of approximately 400 patients.
Dr. Kaiser: How many of our panelists do combined surgery like Dr. Murray did?
Dr. Weng: I do, but it depends on which setting I’m in. I split my time between the county hospital and my university faculty practice. At county, we do combined surgery frequently and I really like it. I would do it more often in my faculty practice if it weren’t for some of the political implications. Single-surgeon combined surgery is much more popular in Europe and elsewhere abroad, and I think it can be a great option. We know that vitrectomy accelerates cataract progression. Many patients start off with some degree of cataract anyway, so it’s nice to be able to take care of both pathologies at once, especially in conditions like an ERM where patients begin to appreciate visual improvement pretty soon after surgery. When they lose it again due to a growing cataract that requires a second surgery, it can be frustrating for them. Like Dr. Murray did, I also like to put in trocar-cannulas before I start the cataract extraction to avoid putting the eye through that transient pressure up to 70 mmHg after you have open wounds. And I always suture the corneal wound with 10-0 nylon before starting the vitrectomy. No matter how good my corneal triplanar wound is, I like the extra security. Beautiful case, Dr. Murray.
Dr. Khanani: I’m in a multispecialty practice and we go to a private hospital. They give us 1 day a week where my partner can come and do 2 cases, so I’m limited in capacity in terms of doing combined cases, but I actually love them. In Reno, the elevation is high, and many patients who want to travel get oil, so we remove oil and cataract at the same time so they don’t have to go through 2 additional surgeries after vitrectomy. Also, there is the issue of physicians being paid half for a combined surgery. So, for me, it’s difficult logistically and in terms of payment, but if I could do combined surgery more often, I would.
Dr. Kaiser: I couldn’t agree more. I do all of my macular cases combined. In macular hole surgery, combined surgery prevents holes from reopening when cataract surgery is done secondarily. I always have a cataract surgeon perform the cataract surgery. I ask them to make a small capsulorhexis and suture the wound, it makes life a lot easier and prevents the lens from moving if we need to use gas.
Dr. Vajzovic: I echo all those comments. I’m in a university setting, and as such I have the luxury of a cornea colleague right next door. For logistical reasons, it ends up being easier to perform combined surgery from a reimbursement standpoint, including reimbursement for surgical packs. Regarding corneal wound closure, I always prefer to close those with 10-0 nylon suture. I have been convinced in the past by a cornea colleague to try sealing the wound with glue instead of suturing, and I have been burned. Therefore, I do suture the corneal wound with nylon before we focus on posterior segment of the eye, particularly as we are not paying attention to the front of the eye when we operate posteriorly. We often change infusion pressures and rotate or depress the eye during surgery. That can translate to corneal wound alterations and potentially loss of fluid.
Dr. Khanani: Dr. Murray, your approach was fantastic and I’ve never seen that. I’ve only seen colleagues do a biopsy and then radiation. Why do what you did instead?
Dr. Murray: For tumors that are smaller than 2.5 mm in thickness, tumor control is 100%.2 The bar is set high with either plaque brachytherapy or proton beam radiotherapy. However, when you use radiotherapy to ablate this tumor, the risk of radiation-associated issues, such as radiation retinopathy, radiation optic neuropathy, or anterior segment neovascular alterations with glaucoma, is incredibly high. We’ve shown, and others have shown, that you can treat those complications with anti-VEGF, and now we have an approach that couples the anterior-segment and posterior-segment surgical procedures into 1 procedure.3-7
This patient’s visual acuity was 20/20- at 6 weeks. There is no plaque brachytherapy patient who is 20/20- with a progressive cataract for 6 months to a year until you operate on them again. When you operate the second time, if you don’t address the posterior pathology at the same time, patients often have an epiretinal membrane or some degree of macular edema. So for small tumors only, in the hands of an ocular oncologist that is a skilled vitreoretinal surgeon, I think you’re looking at the future. I’ve personally been doing this for a decade. And we’ve reported extensively, so there are good data now.3,4 But, if you’re not a vitreoretinal surgeon, this technique is out of your hands.
Dr. Weng: I’d also like to add that during the pandemic, I think we’ve all appreciated the benefit of doing 2 surgeries simultaneously.
Dr. Murray: Agreed, and it benefits both patients and surgeons to perform surgeries in an outpatient setting for a better patient experience. I’m surprised that we don’t see more people doing this now, but I think the next generation of ocular oncologists will.
Dr. Kaiser: Thank you to the panel for this excellent discussion. RP
REFERENCES
- Parke DW 3rd, Sisk RA, Murray TG. Intraoperative intravitreal triamcinolone decreases macular edema after vitrectomy with phacoemulsification. Clin Ophthalmol. 2012;6:1347-1353. doi:10.2147/OPTH.S34653
- Murray TG, Villegas VM, Bach A, Gold AS. Five-year follow-up of microincisional vitrectomy surgery, endolaser tumor ablation, and gene-expression profiling in small uveal melanoma. J Vitreoret Dis. Published online December 1, 2020. doi:10.1177/2474126420972878
- Murray TG, Latiff A, Villegas VM, Gold AS. Aflibercept for radiation maculopathy study: a prospective, randomized clinical study. Ophthalmol Retina. 2019;3(7):561-566. doi:10.1016/j.oret.2019.02.009
- Murray TG, Latiff A, Villegas VM, Gold AS. Aflibercept for radiation maculopathy (ARM study): year-2 extension of a prospective clinical study. J Vitreoret Dis. October 8, 2020. doi:10.1177/2474126420958894
- Kim IK, Lane AM, Jain P, Awh C, Gragoudas ES. Ranibizumab for the prevention of radiation complications in patients treated with proton beam irradiation for choroidal melanoma. Trans Am Ophthalmol Soc. 2016;114:T2.
- Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26(1):60-66. doi:10.5301/ejo.5000670
- Shields CL, Dalvin LA, Chang M, et al. Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (every 4 months for 2 years) for uveal melanoma: comparison with nonrandomized historical control individuals. JAMA Ophthalmol. 2020;138(2):136–146. doi:10.1001/jamaophthalmol.2019.5132








