Dysfunction and dysregulation of the innate immune system resulting in pathologic inflammation appears to play an important role in the pathogenesis of age-related macular degeneration (AMD). Drusen on the macula, which are comprised of a milieu of lipid and protein components, initiate damage signals that trigger activity within the complement immune system.1 In turn, complement activity spurs inflammation and other immune responses intended to clear the foreign body from the macula. In a healthy, non-genetically predisposed eye, several regulatory mechanisms, including complement factor H (CFH), eventually deactivate that inflammation; however, the aging process, along with genetic polymorphisms, attenuate the effectiveness of anti-inflammatory regulation, thus resulting in the constitutive activity that is characteristic of diseases of chronic inflammation, such as macular degeneration.2
The role of inappropriate activity within the complement cascade in driving AMD-associated pathology is supported by 2 lines of evidence. First, a large number of complement activators, complement components, and complement regulatory proteins have been identified as molecular constituents of drusen, indicating the inability to downregulate the complement cascade in the macula.2 Second, strong associations have been found between AMD and variants of several complement pathway-associated genes, such as complement factor H (CFH), complement factor B (CFB), and complement component 3 (C3).2 These genetic variants prevent downregulation of the complement cascade.
THE COMPLEMENT SYSTEM
As its name implies, the complement system aids (complements) the action of antibodies and phagocytic immune cells in eliminating foreign pathogens and damaged, necrotic, and apoptotic cells.3 Complement activation occurs by one of three pathways. Briefly, the classical pathway is initiated by interactions between pattern-recognition receptors on C1q with antibodies and molecules released by inflammatory processes; the lectin pathway is initiated by binding of polysaccharide or glycoprotein motifs associated with microbes or damaged cells (i.e., host and non-host cells); and the alternative pathway is constitutively active—specifically, naturally occurring hydrolysis breaks an internal bond in complement protein 3 (C3) that sets the stage for a complex cascade of activity that eventually results in an amplifying positive feedback loop (Park 2019). CFH is the main inhibitor of the complement alternative pathway by interactions with C3; under normal physiologic conditions, CFH keeps the spontaneous activation of the alternative pathway in check (Bradley 2011).4
The classical, lectin, and alternative pathways all converge at the point of C3 convertase activation.3 Formation of C3 convertase via any of these pathways cleaves C3 into the anaphylatoxin C3a and C3b, the latter of which has 3 distinct functions: elimination of pathogens and apoptotic cells by phagocytosis; initiation of an amplification loop via binding to factor B; and to serve as a building block for C5 convertase, which spurs additional activity within the lectin and classical pathways.3 In turn, C5 convertase cleaves C5 to C5a (another anaphylatoxin) and C5b, which ultimately results in the downstream formation of membrane attack complex (MAC).
Each of these pathways is regulated through a series of proteins and protein signaling, whereas dysfunction in these regulatory pathways yields inappropriate or constitutive activation, often resulting in damage to healthy cells and tissues.3 The primary mechanisms for complement regulation include degradation of complement components, increase in C3 convertase decay, and modulation of MAC assembly.5 An additional mechanism for regulation has been identified in the alternative pathway, where host cell membrane-bound sialylated glycans serve to discriminate host from nonhost cells.5 The recognition of these glycan signals leads to modulation or shutdown of the active immune processes. Notably, while all 3 pathways are understood to be relevant to AMD pathogenesis, activity within the alternative pathway appears to play a crucial role.5
HITS AND MISSES IN HUMORAL INNATE IMMUNITY (COMPLEMENT) THERAPEUTIC TARGETING
Multiple agents targeting various aspects of complement system activity in AMD are in development. However, despite their significant promise, some drug candidates have failed to demonstrate efficacy. For example, targeting of C5, a linkage in the pathway that yields MAC formation, has been attempted with eculizumab, a humanized anti-C5 antibody (Alexion Pharmaceuticals Inc.) and LFG316, a fully human anti-C5 antibody (Novartis International AG);6 however, both failed to demonstrate reduction in geographic atrophy (GA) in phase 2 trials.7,8
Lampalizumab, a humanized antigen binding fragment targeting complement factor D showed positive results in a phase 2 trial but failed to meet its primary endpoint in a phase 3 study of mean reduction in GA lesion area at 48 weeks compared to baseline vs sham injection.9 Finally, CLG561 (Alcon/Novartis), a humanized Fab targeting properdin, which functions to stabilize C3bBb to potentiate cleavage of C3, completed a phase 2 study designed to assess safety and potential efficacy as monotherapy or combined with the anti-C5 molecule LFG316; results have not been published, but neither monotherapy nor combination therapy appeared to achieve significant reduction in GA compared to sham.8
Despite these setbacks, interest in potential complement-targeting molecules has not diminished. Indeed, it may be the case that so-called failures of prior clinical trials may in part be attributable to trial design — proving efficacy vis-a-vis reduction in GA, although a necessary aspect of regulatory review, has proven exceedingly challenging in clinical trials conducted to date. Nevertheless, a number of promising molecules are under development for targeting the complement system, including AAVCAGsCD59, a gene therapy to increase the soluble form of CD59 to inhibit the formation of the membrane attack complex (Hemera Biosciences); GT0005, a subretinal gene therapy in CFI variants to deliver a gene encoding for CFI (Gyroscope Therapeutics); NGM621, an intravitreal anti-C3 antibody (NGM Biopharmaceuticals); FB-LRx, an intravitreal antisense oligonucleotide RNA targeting CFB (Ionis Pharmaceuticals); GEM103, an intravitreal recombinant CFH targeting patients with a CFH common variant (Gemini); and ANX007, an intravitreal Fab that binds C1q to inhibit the classic pathway (Annexon).
In addition, there are 2 promising molecules that have entered later stage clinical development. Pegcetacoplan (APL-2; Apellis Pharmaceuticals), is a pegylated cyclic peptide inhibitor of complement C3. The latter is associated with strong scientific rationale, because C3 is a common point of convergence for all 3 complement pathways. In the phase 2 FILLY study, APL-2 was generally well tolerated, but there was an increase in exudation in treated patients vs sham. After 12 months of follow-up, patients treated with a monthly protocol experienced a 29% reduction in the growth of GA lesion area and there was a 20% reduction among those treated with an every-other-month protocol.10 Based on these findings, the drug’s sponsor initiated 2 phase 3 trials (DERBY and OAKS) with results anticipated later in 2021. The second molecule is the C5 inhibitor avacincaptad pegol (Zimura; Iveric bio). C5 antagonism may have some benefits relative to C3, because doing so may confer the ability for some homeostatic mechanisms of the innate immune system occurring upstream at C3 to remain active. A phase 2b/3 study (GATHER) of avacincaptad pegol reported positive results: 27.38% reduction in the mean rate of GA growth over 12 months in individuals treated with a 2-mg dose and 27.81% for the Zimura 4-mg group, both compared to sham. Similar to APL-2, a greater rate of dose-dependent exudation was reported in treated vs sham patients. A confirmatory, pivotal phase 3 trial (GATHER2) of avacincaptad is under way.
A POTENTIAL ROLE FOR GLYCAN TARGETING
Each cell type of the innate immune system exhibits membrane-bound, glycan-based pattern-recognition receptors (PRRs) that function to recognize damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively), thereby initiating the relevant pathways that give rise to complement activity.11 In the pathobiology of AMD, presence of drusen leads to immune cell recognition of DAMPs, prompting inflammation to clear the foreign substance, but its accumulation and propagation sometimes leads to constitutive activation (Figure 1). This inappropriate inflammatory activity is a hallmark driver of associated pathology. More specifically, macrophages, the main cells of the innate immune system, appear to play a vital role, as they possess the ability to polarize (change functional state) to a phagocytic or a VEGF-secreting state based on conditions in the microenvironment.
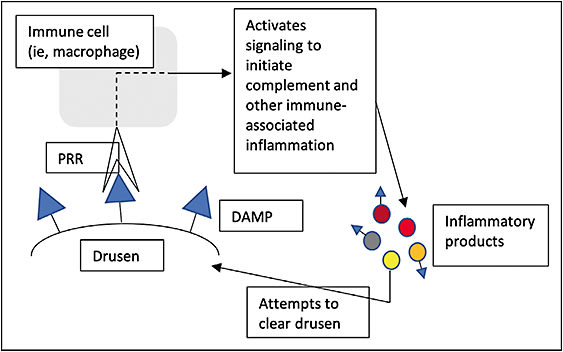
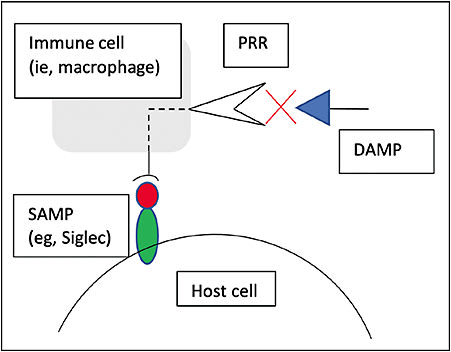
Varki and colleagues described a third class of recognition patterns called self-associated molecular patterns (SAMPs) that are recognized by self-associated molecular pattern-recognition receptors (SPRRs), resulting in modulation or deactivation of innate immune cell activity.12,13 Of note, CFH is a type of SPRR that deactivates the complement cascade; in addition, the largest family of SPRRs receptors, known as sialic acid-binding immunoglobulin-type lectins, or Siglecs, deactivate or prevent activation of innate or adaptive immune cells.11,12 Siglec receptors are responsible for opposing inflammatory cell activation by PRRs and represent the immune system’s predominant intrinsic mechanism for immune regulation (Figure 2).
Aviceda Therapeutics has developed a high throughput screen to identify sialic acid-glycan ligands that bind Siglecs with high affinity; these ligands can then modulate immune cell activity. Ultimately, the goal with such glycol-immune therapeutics is to engage the appropriate immune checkpoint, with the promise to affect all aspects of innate immune function in AMD pathology, including polarization of macrophages to a resolution state, which has the potential benefit of slowing VEGF production, halting phagocytic activity, and reducing cytokine production; shutdown of CFH activation; and inhibition of the inflammasome pathway.
AVD-104 (Aviceda Ophthalmics) is an optimized sialic acid-coated nanoparticle that can bind Siglecs but also has the added property of being able to downregulate the complement cascade by disrupting C3 amplification. The binding of macrophage Siglecs polarizes macrophages to a resting state. The capability of AVD-104 to resolve complement, phagocytic, and angiogenic inflammation with one therapeutic is a promising strategy.
CONCLUSION
It is not surprising that research on potential therapeutics targeting aspects of the innate immune system remains highly active given the lack of treatment for both dry AMD and GA. Despite some setbacks in complement targeting, all of which underscore the challenges in demonstrating efficacy in advanced dry AMD, several promising candidate molecules are in later stages of development. The strategy of targeting immune regulation via glycan-presenting nanoparticles is another approach in development that targets both humoral (complement cascade) and cellular innate immune activity. This global innate immune targeting has the potential to immune-normalize treated patients, which suggests the plausibility of not just slowing progression, but maybe even yielding regression of existing pathology to some degree. Overall, if even one or a handful of these strategies successfully navigates through to clinical availability, it will represent a significant milestone for the treatment of AMD. RP
REFERENCES
- Park DH, Connor KM, Lambris JD. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front Immunol. 2019;10:1007. Published 2019 May 15. doi:10.3389/fimmu.2019.01007
- Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95-112. doi:10.1016/j.preteyeres.2009.11.003
- Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128(3):349-358. doi:10.1001/archophthalmol.2010.18
- Bradley DT, Zipfel PF, Hughes AE. Complement in age-related macular degeneration: a focus on function. Eye (Lond). 2011;25(6):683-693. doi:10.1038/eye.2011.37
- Maugeri A, Barchitta M, Mazzone MG, Giuliano F, Agodi A. Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine. Biomed Res Int. 2018;2018:7532507. Published 2018 Aug 26. doi:10.1155/2018/7532507
- Intravitreal LFG316 in patients with age-related macular degeneration (AMD). ClinicalTrials.gov identifier: NCT01527500. Updated January 5, 2021. Accessed March 8, 2021. https://clinicaltrials.gov/ct2/show/NCT01527500
- Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693-701. doi:10.1016/j.ophtha.2013.09.044
- CLG561 proof-of-concept study as a monotherapy and in combination with LFG316 in subjects with geographic atrophy (GA). ClinicalTrials.gov identifier: NCT02515942. Updated May 30, 2019. Accessed March 9, 2021. https://clinicaltrials.gov/ct2/show/results/NCT02515942
- Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136(6):666-677. doi:10.1001/jamaophthalmol.2018.1544
- Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186-195. doi:10.1016/j.ophtha.2019.07.011
- Varki A. Letter to the Glyco-Forum: Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21(9):1121-1124, https://doi.org/10.1093/glycob/cwr087
- Crocker PR, Varki A. Siglecs in the immune system. Immunology. 2001;103(2):137-145. doi:10.1046/j.0019-2805.2001.01241.x
- Läubli H, Varki A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci. 2020;77(4):593-605. doi:10.1007/s00018-019-03288-x








