The most common cause of central vision loss in the developed world is leaking and bleeding due to the development of subretinal neovascularization associated with exudative age-related macular degeneration (AMD). This abnormal leaking and bleeding is typically found in and under the retina, as well as below the retinal pigment epithelium (RPE), and is caused by choroidal neovascularization (CNV) associated with exudative AMD. An important subtype of exudative AMD includes CNV in which subretinal aneurysmal dilations are a part of the lesion. This entity has been called polypoidal choroidal vasculopathy (PCV) or subretinal aneurysmal neovascularization.1,2
This article will discuss why this subtype is often overlooked and will emphasize the importance of diagnosis, as well as the different diagnostic techniques used to make the diagnosis. Identifying PCV is clinically very important, because the diagnosis of PCV guides therapy and often will allow better visual and anatomic results using alternative therapies as opposed to the current standard of anti-vascular endothelial growth factor (anti-VEGF) monotherapy injections.
ETIOLOGY AND PRESENTATION
Polypoidal choroidal vasculopathy was initially theorized to be a choroidal vascular abnormality.1,2 However, optical coherence tomography (OCT) studies have localized the lesion between Bruch’s membrane and the RPE.3,4 In the Gass anatomic classification of subretinal neovascularization,5 this would be a type I subretinal neovascularization under the RPE and above Bruch’s membrane. Type II subretinal neovascularization occurs above the RPE and in the subretinal space.5 Type III neovascularization or retinal angiomatous proliferation includes an intraretinal component.5 Polypoidal choroidal vasculopathy can also have type II components above the RPE, so it is not always type I.
The clinical presenting features of this aneurysmal form of CNV look very similar to what is seen with typical exudative AMD. There is subretinal fluid and blood, as well as associated subretinal exudate and retinal pigment epithelial detachment (RPED). Polypoidal choroidal vasculopathy has some distinguishing clinical features:6 more subretinal fluid, higher height of subretinal fluid, more RPED, less intraretinal fluid or macular edema, and higher frequency of subretinal hemorrhage. However, unlike with typical exudative AMD, the PCV diagnosis cannot be made on purely fundus examination and fluorescein angiography (FA).7 Fluorescein angiography in PCV for the majority of cases shows occult leakage or occult CNV often associated with RPED, which looks similar to typical exudative AMD. Because of these similarities, PCV often is not diagnosed without further testing beyond the usual diagnostic studies for exudative AMD. Further studies include indocyanine green angiography (ICGA), B scan optical OCT for PCV, en face OCT, and OCT angiography (OCTA) (Table 1).
| DIAGNOSTIC TESTING METHOD | IMAGING FEATURES |
|---|---|
| Fundus examination and fundus photos | Subretinal reddish branching network or reddish polypoidal lesion |
| Fluorescein angiography | Usually occult subretinal neovascularization with occult diffuse leakage not distinguishable from typical exudative AMD |
| Indocyanine green angiography (relatively early phase images at 3 to 5 minutes) | Polypoidal lesions with branching vascular network Occasionally pulsatile polypoidal lesion |
| B scan OCT | Polypoidal lesions image as an inverted U-shaped elevation of the RPE Branching vascular network images as a double line sign (low lying linear elevation of the retinal pigment epithelium [RPE]) Ring-shaped lesion beneath the RPE |
| En face OCT (RPE to RPE fit, or outer retina to choriocapillaris cuts) | Polypoidal subretinal neovascularization network with branching vascular network and polypoidal dilations |
| Indocyanine green angiography | Subretinal neovascularization with polypoidal dilations Polypoidal dilations with current available technology not seen as well as indocyanine green angiography |
DIAGNOSIS
Indocyanine green angiography is the gold standard for making the diagnosis of PCV, and it is best visualized with the scanning laser ophthalmoscope (SLO; options available from Heidelberg, Optos, and Nidek). On ICGA, it is important to look at images relatively early after injection. Usually, the best images of PCV lesions are visualized 3 to 5 minutes after ICG dye injection. Polypoidal choroidal vasculopathy has been diagnosed with high prevalence in Asian populations.8,9 However, PCV in White populations has been underdiagnosed due to a lack of access to ICGA or lack of interest in ICGA diagnosis of PCV.9-13
Polypoidal choroidal vasculopathy is much more prevalent than generally recognized. Although most ophthalmologists think it is a disease predominant in Asian patients, where prevalence has been noted to be as high as 50% or more in eyes presenting with exudation and bleeding from subretinal neovascularization,8 PCV has now been recognized in approximately 25% of White patients,9-13 and in most Black patients with exudative AMD. Early studies of AMD in White patients involved fundus photo ICGA, which is much less sensitive at detecting PCV than ICGA utilizing the scanning laser ophthalmoscope (SLO).14 When SLO ICGA was utilized in studies of White patients with investigators experienced in reading ICGA for PCV, the prevalence of PCV in exudative AMD in White patients ranged from 20% in the PERSIST study from Duke,11 to 24.5% in a predominantly European ancestry study from Brazil,12 and to as high as 31% in a study of White patients from Hawaii, where many of the White patients came from a variety of states in the United States.13 Thus, PCV is commonly seen in many practices, but is under-recognized due to lack of experience in making the diagnosis, especially with ICGA. Although typical exudative AMD has long been known to be more frequent in females, PCV has a strong male predilection in Asian patients, and does not show the usual female predominance of typical exudative AMD in White patients.8,9 Polypoidal choroidal vasculopathy lesions in Black patients often have larger caliber vessels and are more often peripapillary.
Another modality capable of assisting in the diagnosis of PCV is B scan OCT. A B scan OCT shows the polypoidal lesions as inverted U-shaped elevations of the RPE with steep borders and a gradual slope at the top of the RPE elevations.3,4 The branching vascular network (BVN) is seen as a “double line sign,” which represents a slight elevation of the RPE above Bruch’s membrane by the vascular network.15 The usual B scan OCT on most spectral-domain OCT (SD-OCT) machines is available in most practices, and can be helpful to diagnose PCV by imaging the inverted U-shaped elevation of the RPE with heterogeneous reflectivity.3,4 The polypoidal lesions can be imaged on B scan OCT in PCV cases confirmed on ICGA with the SLO in 57% of cases.16 However, these lesions are decreased after anti-VEGF therapy, so if B scan OCT is used to diagnose PCV in an anti-VEGF-resistant case, it is important to look at the B scan prior to treatment because previously imaged polypoidal lesions will be lost in 56% of cases.16 A recent study by the Asia Pacific Ocular imaging Society showed that SD-OCT findings can a have high degree of accuracy of diagnosing PCV when using 3 criteria: (1) a ring-shaped lesion underneath the RPE, (2) en face OCT complex RPE elevation, and (3) a sharp-peaked inverted U-shaped PED with an accuracy of 82%.17
Findings other than the imaging of polypoidal lesions alone are also helpful to suspect the diagnosis of PCV. Retinal pigment epithelial detachment is a frequent finding associated with PCV,4,6,12-13 and the RPED may mask the aneurysmal lesions, especially with the usual hypofluorescence and decreased visibility of hyperfluorescent lesions within the RPED when using the SLO. Polypoidal choroidal vasculopathy lesions often appear at the edge of the RPED or at a notch in the RPED (Figure 1). There is often a BVN with a double line sign connecting to the polypoidal lesions (Figure 2).14

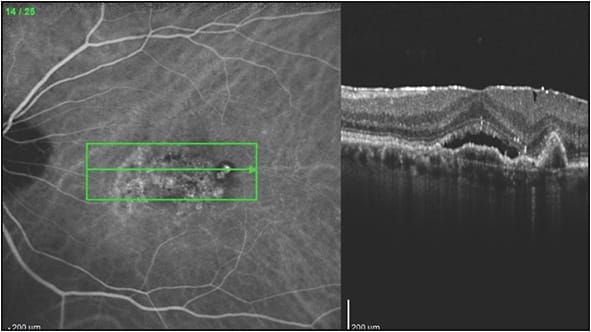
As stated previously, the ICGA SLO is the most sensitive ICGA that can best visualize polypoidal lesions and the BVN, which makes it more suitable for diagnosing PCV than flash fundus camera ICGA.15 The use of flash camera ICGA alone is a reason that many practices feel that they do not see PCV often in their patients (our practice includes flash camera ICGA at some offices, and SLO ICGA in our main office, and the differences in sensitivity are striking). Fluorescein angiography and ICGA can be performed at the same time on the SLO, but separate images should be taken because the focal points of the FA are more superficial, and those of the ICGA are deeper.
DIAGNOSTIC CHALLENGES
Indocyanine green angiography is often not available in many clinical settings around the world, including the United States, and when available, ICGA is often not performed, or expertise in reading the ICGA for PCV is not available. If ICGA is not available or not ordered, the diagnosis can be made using multimodality imaging with high specificity but lower sensitivity than ICGA. Optical coherence tomography angiography images the BVN well, although images of the aneurysmal or polypoidal lesions are poorer due to slower blood flow within the polypoidal lesions (Figure 3).18,19
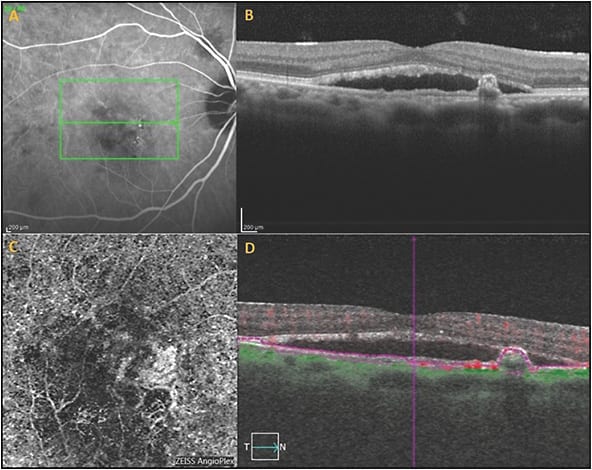
En face OCT imaging also can often demonstrate the BVN and the aneurysmal dilations associated with PCV anatomically, but is again less sensitive than ICGA.18,20,21 However, lesions on en face OCT may appear larger than the CNV on ICGA, and this may be because the en face OCT images the whole vascular complex and the extracellular matrix surrounding the PCV complex, which may also elevate the RPE (Figure 4).18,20,21 Correlation of OCTA and en face OCT with B scan OCT increases the specificity of the diagnosis of PCV,18,19 because polypoidal lesions appear as inverted U-shaped lesions with heterogeneous reflectivity,3-4 whereas the BVN appears as a shallow elevation of the RPE above Bruch’s membrane (double line sign).15

There are no genetic markers or well-known phenotypic markers for anti-VEGF resistance. However, there is one phenotypic marker that does predict anti-VEGF resistance, and that is subretinal aneurysmal lesions in the CNV complex of PCV. While this was first recognized in case studies,22 subsequent studies have confirmed a significantly higher rate of persistent disease activity in eyes with PCV when treated with the currently available anti-VEGF agents.9,20 This was seen in a study of PRN treatment in Sweden with ranibizumab.20 This was also seen in a retrospective study in the United States, which defined anti-VEGF resistance as lack of clinical response after four consecutive anti-VEGF injections and showed a statistically significant higher prevalence of PCV in eyes with anti-VEGF resistance as opposed to typical exudative AMD without PCV.9 In addition, this was significant in both Asian and White patients specifically, and the difference between the 2 groups was actually greater in the White exudative AMD group.9
HOW DIAGNOSIS OF PCV AFFECTS TREAMENT
Based on the EVEREST II study, it is reasonable to offer either anti-VEGF therapy or combination PDT and anti-VEGF injection for PCV.24-26 Practically, if vision is still very good at 20/40 to 20/50 or better, then it is reasonable to start with anti-VEGF therapy. However, if vision is 20/50 to 20/60 or worse, it is also very reasonable to start with combination PDT and anti-VEGF therapy, because results in the EVEREST II study were better in regards to treatment burden (6 injections vs 12 injections over 2 years)25-26 and visual recovery for combination PDT with anti-VEGF treatment for PCV than anti-VEGF injection monotherapy.25-26 In the EVEREST II study there were no cases of sudden vision loss after PDT. However, when treating with PDT, there is a potential risk of choroidal ischemia or subretinal hemorrhage, so this small risk may be avoided in eyes with good vision. Interestingly, the risk of choroidal ischemia may be less in PCV due to the associated thicker choroid in these cases. If lesions are extrafoveal and the PDT lesion spot can avoid the fovea, it is very reasonable to begin with combination PDT and anti-VEGF therapy with full fluence.
The treatment of exudative AMD with PDT began in the early 2000s, when FA was used to determine lesion size and the area of leakage was encircled. The greatest linear dimension was calculated based on leakage from the FA. The initial recommendation was to use a treatment spot that is 1,000 µm larger than the greatest linear dimension of leakage on the FA for typical exudative AMD, so this resulted in a very broad area of treatment with PDT. Treatment for PCV lesions is very different, as the PCV spot size is based on the ICGA, not the FA. The PCV spot size typically matches the actual size of the PCV vascular complex, but it is also reasonable to add a small 300-µm border around the greatest linear dimension of the lesion on ICGA.
For verteporfin (Visudyne; Bausch + Lomb), an intravenous dose of 6 mg per square meter of body surface area is given and the diode laser (689 nm) is directed to the treatment area 15 minutes after intravenous infusion of dye.24,26 If the PCV lesion is extrafoveal, we recommend full fluence PDT (50 J/cm2 of light at 600 mW/cm2 for 83 seconds). If the lesion is subfoveal, and vision is good, then reduced fluence PDT (25 J/cm2 of light at 300 mW/cm2) can be considered. Laser spot duration is 83 seconds for both full-fluence and reduced-fluence treatment, but the settings of the laser are different as noted above. If vision is 20/50 to 20/60 or worse, full-fluence treatment is reasonable for subfoveal lesions, based on the EVEREST II study. Intravitreal dexamethasone may also be helpful in combination PDT, because PDT itself is a treatment that causes inflammation.
CASE EXAMPLE OF PRIMARY COMBINATION PDT
An 85-year-old male presented with blurred vision in the right eye for 5 months. Visual acuity was 20/60 and there was RPED, serous retinal detachment, pachydrusen, and subretinal exudates. Indocyanine green angiography revealed a BVN and polypoidal aneurysmal lesions in the superotemporal macula (Figure 5). The patient refused starting frequent intravitreal anti-VEGF therapy, and specifically requested a therapy that could minimize treatment burden and eye injections. He underwent full fluence PDT targeting the lesion identified on the ICGA with intravitreal bevacizumab 1.25 mg and dexamethasone 400 µg. The lesion dramatically responded to the treatment. There was slight residual RPED and subretinal fluid, but after 2 subsequent intravitreal injections of 1.25 mg bevacizumab and 400 µg dexamethasone, the RPED resolved with resolution of subretinal fluid and exudate (Figure 6). His vision recovered to 20/40 and there was no need for further injections or PDT for the rest of his life, as he passed away 6 years after the combination PDT and intravitreal bevacizumab combined with dexamethasone. If an eye with exudative AMD has already been treated with standard-of-care anti-VEGF injections, and there is persistent disease activity, combination PDT and anti-VEGF therapy may be very helpful in decreasing treatment burden and improving anatomic results.
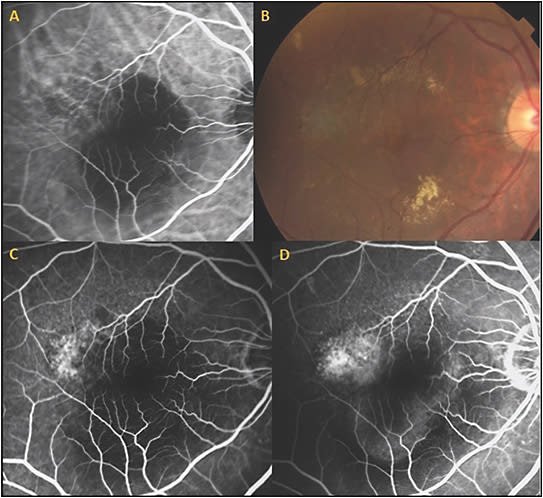
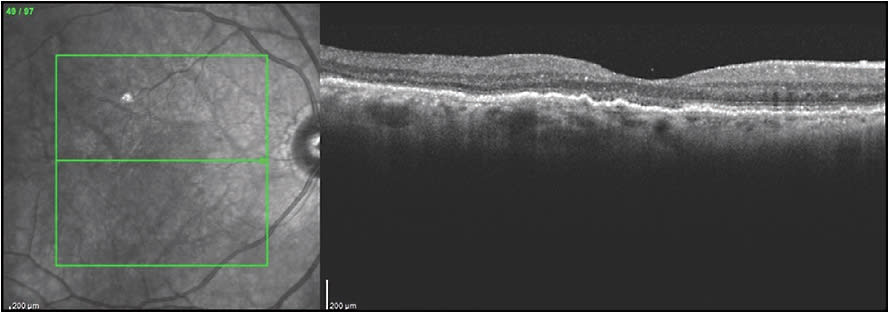
CASE EXAMPLE OF COMBINATION PDT AFTER PREVIOUS ANTI-VEGF THERAPY
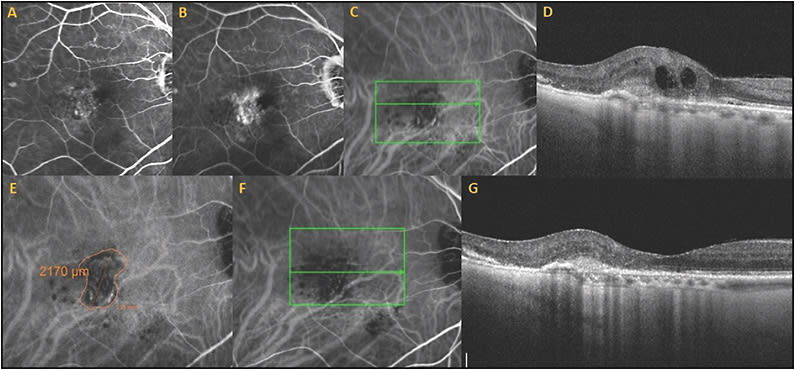
This patient is a 96-year-old male who was diagnosed with exudative AMD with a vascularized RPED, subretinal fluid, macular cystic changes, and subretinal hyperreflective material. Fluorescein angiography showed occult leakage. He started intravitreal aflibercept injections but there was persistent subretinal fluid, subretinal hemorrhage, RPED, and subretinal exudates, despite 6 monthly treatments. The patient was already frustrated by the frequent visits and was considering stopping therapy.
Due to persistent disease activity, ICGA was performed, and the diagnosis of the polypoidal subtype of exudative AMD was made. This ICG was utilized to guide PDT treatment and to measure the spot size (Figure 7). Vision was 20/40, and the lesion was subfoveal, so reduced-fluence PDT was performed, but there was persistent leakage and vision of 20/60. Three months after the first PDT, a second full-fluence PDT was then performed combined with same-day injection of bevacizumab 1.25 mg and dexamethasone 400 µg. There was marked resolution of the RPED, macular edema, and subretinal fluid, and he now requires aflibercept injections only every 3 months with complete resolution of RPED, subretinal fluid, and persistent disease activity (Figure 7). In a recent visit, the patient’s vision recovered to 20/40. The excellent vision and decreased treatment burden were extremely beneficial for this patient.
DISCUSSION
Thermal focal macular laser photocoagulation can play a useful role in the management of PCV. If the diagnosis of PCV is made with extrafoveal polypoidal lesions resulting in leakage, focal macular laser treatment of the lesions can be considered with or without supplemental anti-VEGF therapy. This may result in long-term stabilization of the leakage with good vision and without the need for long-term continuing treatment and higher treatment burden. The goal of thermal laser is to close the polypoidal lesion and prevent further leakage or bleeding, while protecting the fovea from PCV-induced damage or laser photocoagulation-induced damage.27
Finally, PCV may be more responsive to certain anti-VEGF medications, because aflibercept is the treatment of choice in Asia for PCV and aflibercept has shown a significantly better response in some eyes treated previously with other currently utilized anti-VEGF agents.28,29 In the randomized studies that included PCV eyes there was also evidence that brolucizumab had a better drying effect than aflibercept, although there are the recent concerns of inflammation and vasculitis when treating with brolucizumab. Thus, the anti-VEGF agents in order of most significant response in the PCV subtype of exudative AMD are brolucizumab, followed by aflibercept, then ranibizumab, and lastly bevacizumab. 28,29 The PLANET study confirmed the efficacy of aflibercept in the treatment of PCV but was not a true comparison study of whether or not PDT is useful in the treatment of PCV. This study compared a group with 100% of patients treated with aflibercept only to a group with 85% treated with aflibercept only. Only 15% of the PDT group actually received PDT, and most only had 1 PDT treatment based on the criteria for treatment in the PLANET study.29
CONCLUSION
PCV is the most important subtype of exudative AMD, because it provides a marker for anti-VEGF resistance, which may affect therapeutic planning for treatment-naïve eyes as well as eyes responding poorly to anti-VEGF therapy. Combination PDT and anti-VEGF therapy can significantly decrease treatment burden and improve anatomic and visual outcomes. Although ICGA with the SLO is the gold standard for diagnosing PCV, retina specialists should use all modalities, including B scan OCT, en face OCT, and OCTA, to make the diagnosis of PCV (Table 1), and these modalities will continue to improve. Polypoidal choroidal vasculopathy is not only common in Asian patients but is more common than previously recognized in exudative AMD in White patients (25%), indicating that it makes up a very significant part of the patients presenting with exudative AMD throughout the world. RP
REFERENCES
- Yannuzzi LA. Idiopathic choroidal vasculopathy. Presented at: The Macula Society Annual Meeting, February 5, 1982; Miami, Florida.
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8.
- Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012;32(6):1057-1068. doi:10.1097/IAE.0b013e31823beb14
- Kokame GT. Prospective evaluation of subretinal vessel location in polypoidal choroidal vasculopathy (PCV) and response of hemorrhagic and exudative PCV to high-dose antiangiogenic therapy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:74-93.
- Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4th ed. St. Louis: Mosby; 1997.
- Ozawa S, Ishikawa K, Ito Y, et al. Differences in macular morphology between polypoidal choroidal vasculopathy and exudative age-related macular degeneration detected by optical coherence tomography. Retina. 2009;29(6):793-802. doi:10.1097/IAE.0b013e3181a3b7d9
- Kokame GT. Polypoidal choroidal vasculopathy--an important diagnosis to make with therapeutic implications. Retina. 2012;32(8):1446-1448. doi:10.1097/IAE.0b013e3182695bf8
- Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008;19(3):208-212. doi:10.1097/ICU.0b013e3282fb7c33
- Kokame GT, deCarlo TE, Kaneko KN, Omizo JN, Lian R. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744-752. doi:10.1016/j.oret.2019.04.018
- Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238(9):752-759. doi:10.1007/s004170000180
- Mettu PS Allingham MJ, Nicholas PC, Cousins SW. Neovascular morphology by ICG angiography and response to loading-dose anti-vegf therapy in patients with neovascular AMD: results of the PERSIST study. Invest Ophthalmol Vis Sci. 2016;57(12).
- Pereira FB, Veloso CE, Kokame GT, Nehemy MB. Characteristics of neovascular age-related macular degeneration in Brazilian patients. Ophthalmologica. 2015;234(4):233-242. doi:10.1159/000439359
- Kokame GT, Liu K, Kokame KA, Kaneko KN, Omizo JN. Clinical characteristics of polypoidal choroidal vasculopathy and anti-vascular endothelial growth factor treatment response in Caucasians. Ophthalmologica. 2020;243(3):178-186. doi:10.1159/000503834
- Cheung CM, Lai TY, Chen SJ, et al. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: the group experience with digital fundus photography and confocal scanning laser ophthalmoscopy. Retina. 2014;34(12):2397-2406. doi:10.1097/IAE.0000000000000255
- Saito M, Iida T, Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina. 2008;28(3):459-464. doi:10.1097/IAE.0b013e318156db60
- Kokame GT, Omizo JN, Kokame KA, Yamane ML. Differentiation between typical exudative age related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV) using B-scan optical coherence tomography (OCT) with decreased findings after antiangiogenic treatment. Presented at RS2020VR, the virtual 2020 Annual Meeting of the Retina Society, July 2020.
- Cheung CMG, Lai TYY, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup. Ophthalmology. 2021;128(3):443-452. doi:10.1016/j.ophtha.2020.08.006
- de Carlo TE, Kokame GT, Kaneko KN, Lian R, Lai JC, Wee R. Sensitivity and specificity of detecting polypoidal choroidal vasculopathy with en face optical coherence tomography and optical coherence tomography angiography. Retina. 2019;39(7):1343-1352. doi:10.1097/IAE.0000000000002139
- de Carlo TE, Kokame GT, Shantha JG, Lai JC, Wee R. Spectral-domain optical coherence tomography angiography for the diagnosis and evaluation of polypoidal choroidal vasculopathy. Ophthalmologica. 2018;239(2-3):103-109. doi:10.1159/000481540
- Kokame GT, Hirai K, Yanagihara R. Polypoidal choroidal vasculopathy: imaging by indocyanine green angiography and en face optical coherence tomography. JAMA Ophthalmol. 2015;133(11):e151886. doi:10.1001/jamaophthalmol.2015.1886
- Sayanagi K, Gomi F, Akiba M, Sawa M, Hara C, Nishida K. En-face high-penetration optical coherence tomography imaging in polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99(1):29-35. doi:10.1136/bjophthalmol-2013-304658
- Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148(1):70-8.e1. doi:10.1016/j.ajo.2009.02.012
- Hatz K, Prünte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98(2):188-194. doi:10.1136/bjophthalmol-2013-303444
- Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135(11):1206-1213. doi:10.1001/jamaophthalmol.2017.4030
- Kokame GT, Kim JE. Treatment for a Subtype of Exudative Macular Degeneration-Another Mountain Climbed. JAMA Ophthalmol. 2020;138(9):942-944. doi:10.1001/jamaophthalmol.2020.2421
- Lim TH, Lai TYY, Takahashi K, et al. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: The EVEREST II randomized clinical trial. JAMA Ophthalmol. 2020;138(9):935-942. doi:10.1001/jamaophthalmol.2020.2443
- Kokame GT. Polypoidal choroidal vasculopathy - a type I polypoidal subretinal neovasculopathy. Open Ophthalmol J. 2013;7:82-84. doi:10.2174/1874364101307010082
- Kokame GT, Lai JC, Wee R, et al. Prospective clinical trial of Intravitreal aflibercept treatment for PolypoIdal choroidal vasculopathy with hemorrhage or exudation (EPIC study): 6 month results. BMC Ophthalmol. 2016;16:127. Published 2016 Jul 27. doi:10.1186/s12886-016-0305-2
- Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial [published correction appears in JAMA Ophthalmol. 2018 Jul 1;136(7):840]. JAMA Ophthalmol. 2018;136(7):786-793. doi:10.1001/jamaophthalmol.2018.1804








