Brolucizumab, a new anti-VEGF therapy for wet age-related macular degeneration (AMD), was approved by the FDA in late 2019 (Beovu; Novartis). Shortly after, with the help of the American Academy of Ophthalmology IRIS Registry (Intelligent Research in Sight) data curation and analytics partner, Verana Health, my colleagues and I sought to evaluate real-world demographic and clinical characteristics of wet AMD patients who started intravitreal injection therapy with this new drug.1
The researchers relied on data from the IRIS Registry, which contains records for more than 360 million patient visits, including more than 1.7 million unique patients who received anti-VEGF injections during the study period. The IRIS Registry includes almost all retina specialists in the United States, from all types of practices, so it provides comprehensive insights into real-world practice patterns. Our analysis was conducted in the Spring of 2020, just a few months after the introduction of Beovu to the market. Therefore, most of the eyes had fewer than 3 months of follow-up after the first brolucizumab injection. Notably, these data were obtained before widespread COVID-related office closures or appointment cancellations and before the safety signal of inflammation affected brolucizumab prescribing.
BASELINE CHARACTERISTICS
In all, there were 10,594 eyes of 9,457 wet AMD patients who received at least 1 brolucizumab injection. The patients had a mean age of 80.5 years; a majority were female (58%) and white (84%). Most (88%) were treated unilaterally. The patients came from all geographic regions of the United States. About two-thirds were covered by Medicare, with another 7% coming from Medicare Advantage plans and 4% from commercial insurance plans.
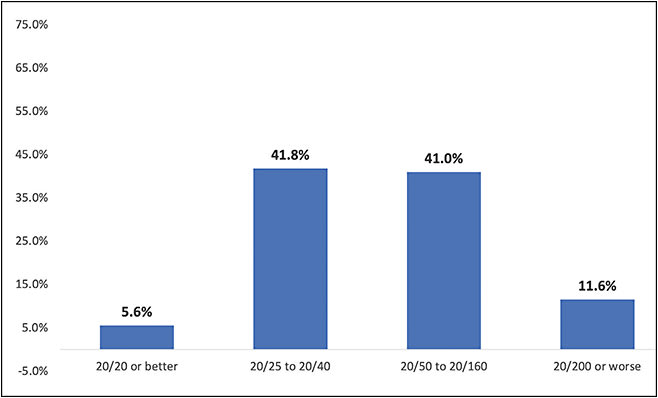
Most of the eyes (83%) had visual acuity in the 20/25 to 20/160 range at baseline, with less than 12% seeing worse than 20/200 (Figure 1). Forty-five percent were pseudophakic, while 35% had cataracts, 18% had glaucoma, and 14% had an epiretinal membrane. Fewer than 3% of the eyes (in each case) had diabetic retinopathy, macular hole, posterior vitreous detachment, retinal vein occlusion, or vitreomacular traction.
Given that brolucizumab had only recently been introduced, it is not surprising that almost all of the eyes studied (91%) had a history of treatment with other anti-VEGF agents during the previous 12 months. The majority of those who switched to brolucizumab had most recently been treated with aflibercept (Eylea; Regeneron) (71%), with the rest about equally split between ranibizumab (Lucentis; Genentech) and bevacizumab (Avastin; Genentech) (Figure 2). About 20% had already tried 2 or more anti-VEGF agents during the past year.

Compared to patients in a 2019 study evaluating injection intervals in treatment-naïve wet AMD patients started on any anti-VEGF therapy,2 this study found that the brolucizumab patients were somewhat more likely to be male or non-White than patients in the earlier study (Table 1). They were also more likely to be treated bilaterally with brolucizumab (12% vs 4.2% treated bilaterally with the same anti-VEGF in the earlier study). This may be because the brolucizumab study was comprised mostly of established rather than treatment-naïve patients, so they were more likely to have bilateral disease. It is possible that doctors were more comfortable treating their established patients bilaterally, even with a very new drug.
| BROLUCIZUMAB* (10,594 EYES OF 9,457 PATIENTS) |
ALL ANTI-VEGF** (56,672 EYES OF 54,392 PATIENTS) |
|
| Patient characteristics | ||
| Mean age (SD) | 80.5 (8.5) | 81.1 (8.4) |
| Female gender | 58.0% | 64.7% |
| White race | 84.2%† | 90.6% |
| Bilateral injection | 12.0% | 4.2% |
| Eye characteristics | ||
| Vision 20/40 or better | 47.4% | 39.1% |
| Vision 20/200 or worse | 11.6% | 23.7% |
| *Brolucizumab injections evaluated in 2020 among wet AMD patients **All anti-VEGF injections evaluated in 2019 among treatment-naïve wet AMD patients † 13.9% classified as “race unknown;” actual percentage of White patients may have been higher |
||
A major difference between the brolucizumab patients in this study and last year’s analysis of all new anti-VEGF patients is that those injected with brolucizumab had better vision at baseline (Table 1). In fact, they were about half as likely (12% vs 24%) to have initial acuity of 20/200 or worse. Although these are separate data sets and not directly comparable, it is an interesting finding.
REASONS TO SWITCH
When we think about this data, it is important to consider why patients might have been switched to the new drug in the first place. Although we cannot determine the clinical reasons from the data itself, common sense suggests some possibilities. One group of switchers likely comprises those who were nonresponders to a previous anti-VEGF injection. If patients still had residual fluid despite maximal therapy, their doctors would be open to switching them to a different agent. We know that 15% of these patients had switched once already before they tried brolucizumab, and these patients were more likely than most to be switched again.
A second group was probably in a very different situation. Their anti-VEGF therapy was working very effectively, but they had probably hit the limits of how long the interval duration could be extended. Pivotal trial data on brolucizumab suggested that patients could be maintained on it at 8- to 12-week intervals,3 so it follows that clinicians seeking to extend the interval to closer to 12 weeks might try to switch patients to the newer drug.
In this brolucizumab study, the mean injection interval for the immediate prior anti-VEGF over the previous 12 months was between 60 days and 76 days, or 8 weeks to 11 weeks. However, the standard deviations were very large, making these mean values less useful. The median interval since the last injection (or an average of the last 2-3 injections) was a very consistent 42 days (6 weeks). One can see how it would be desirable to switch these patients to try to extend dosing intervals to at least 8 weeks and perhaps 12 weeks between injections. This would be especially true among patients with better baseline vision who were probably leading more active lives, and possibly even still working.
Finally, systemic or individual barriers to switching medications may have played some role in physicians’ decision making. The proportion of patients switching from bevacizumab to brolucizumab in our analysis is lower than would be expected by market share alone. Patients on bevacizumab may have been restricted to the less-expensive therapy by their insurance carrier (particularly those with Medicare Advantage or non-Medicare commercial plans) and less able to switch than those already getting a branded injection like aflibercept. We also know that there are some doctors who almost exclusively prescribe one preferred anti-VEGF agent. These doctors may have been less likely to switch when a new drug became available.
There has been much less switching to brolucizumab since the labeling was updated to reflect a higher risk of retinal vasculitis and occlusive events and physicians waited for further safety data on these rare but concerning complications. New evidence has recently emerged that prior intraocular inflammation and/or prior retinal vascular occlusion may be key factors that predispose patients to these inflammatory complications.4,5 Further analysis is ongoing.
What hasn’t changed is that a significant percentage of patients continue to have unresolved fluid, even with monthly injections — something that is often unsustainable over the long term without loss of vision. Retina specialists have a continued need for therapies that can safely extend the treatment interval for patients. Longer lasting anti-VEGF therapies are one way to address this, but there are also emerging port delivery and other sustained-release technologies that hold promise for wet AMD patients in the future.
REAL-WORLD DATA
Large, real-world data sets such as the one analyzed from the IRIS Registry will continue to be an essential part of the effort to understand and influence practice patterns in the treatment of wet AMD. Although controlled clinical trials are the gold standard for evaluating safety and efficacy, they can only have participation of a small sample of patients and doctors, from which we extrapolate the results to the entire population. The IRIS Registry includes nearly all the data, without extrapolation. The depth and breadth of this registry can present challenges, which is why the data connection, curation, and contextualization expertise of an analytics partner can help eliminate missing or incorrectly entered data and structure research queries that truly measure what we want to measure.
In my personal experience working with the IRIS Registry thus far, it adds a level of accuracy beyond what is available from claims data, because it comes from electronic health records. With advances in data science, we can now unlock immense value from electronic health records systems to aggregate patient characteristics, true diagnoses (not just suspected diagnosis codes) and treatment decisions for a more robust analysis. RP
REFERENCES
- MacCumber M, Yu JS, Guruprasad B, et al. Profiles of patients who initiated brolucizumab for neovascular (wet) age-related macular degeneration (AMD) in the IRIS Registry. Presented at: Retina Society Annual Meeting, August, 2020. https://avenue.live/retina-society/presentations/maccumber-mathew-profiles-of-patients.pdf
- MacCumber M, Yu JS, Sagkriotis A, et al. Injection intervals in treatment-naïve neovascular AMD patients who received anti-VEGF agents: an analysis of the IRIS Registry. Presented at: Retina Society Annual Meeting, September, 2019, London.
- Dugel PU, Koh A, Ogura Y, et al; on behalf of the HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84.
- Ip M, Albini T, Zarbin M, et al. The brolucizumab experience thus far: a health economics and outcomes research analysis. Presented at: American Academy of Ophthalmology 2020 Virtual Congress, November 2020.
- Zarbin M, Basavarajaiah G, Yu J, et al. Profiles and early outcomes of patients who initiated brolucizumab for neovascular (wet) age-related macular degeneration (AMD) in the IRIS Registry and Komodo database. Presented at: American Academy of Ophthalmology 2020 Virtual Congress. Session: PO395. November 2020.








