Neovascular age-related macular degeneration (nAMD) is one of the leading causes of irreversible vision loss in patients more than 50 years of age. Characterized by choroidal neovascular membranes (CNVM), which can leak under or within the retina, nAMD can cause rapid sudden vision loss that is irreversible if not treated swiftly and continuously. While previously difficult to treat, the advent of injectable anti-vascular endothelial growth factor (anti-VEGF) drugs have revolutionized the treatment of nAMD. These medications block VEGF to reduce leakage and bleeding from CNVM complexes.
WHERE WE ARE
There are currently multiple options to treat nAMD, ranging from bevacizumab (Avastin; Genentech), ranibizumab (Lucentis; Genentech), aflibercept (Eyelea; Regeneron), and brolucizumab (Beovu; Novartis). Bevacizumab is a humanized recombinant full-sized monoclonal antibody against VEGF-A, and is currently an off-label treatment for nAMD. Ranibizumab is an FDA-approved humanized antibody fragment that also targets VEGF-A. Aflibercept is an FDA-approved recombinant fusion protein of the human VEGF receptor, which targets VEGF-A, VEGF-B, and placental growth factor. Brolucizumab is an FDA-approved single chain antibody fragment, which also targets VEGF-A. Bevacizumab, ranibizumab, and aflibercept can be given as frequently as every 4 weeks, while brolucizumab is initially given every 4 weeks for the first 3 doses, followed by one dose every 8 to 12 weeks.
In the pipeline are other medications and devices, such as faricimab (Genentech), a bispecific antibody that targets angiopoietin-2 and VEGF-A and is given every 8 weeks. Preliminary results from the global Phase 3 TENAYA and LUCERNE studies for faricimab in the treatment of nAMD demonstrated noninferiority to aflibercept.1 Additionally, around 45% of patients undergoing treatment with faricimab were able to be treated with q16 week dosing during the first year, suggesting durability of treatment.
Also in the pipeline is the Port Delivery System (PDS; Genentech), a surgical, reusable drug reservoir of ranibizumab that is placed in the operating room but subsequently refilled in the office. Preliminary results from the Phase 3 ARCHWAY study showed noninferiority compared with monthly ranibizumab eye injections, with about 98.4% of patients going 6 months without needing additional treatment, suggesting increased durability of treatment.2 Both faricimab and the PDS device are currently pending U.S. Food and Drug Administration approval and European Medicines Agency approval.
TREATMENT CHOICES
Focusing on the current treatments used in nAMD, it can be challenging to determine which drug is optimal for your specific patient. The Comparison of AMD Treatments Trials (CATT) was a multicenter noninferiority trial comparing bevacizumab with ranibizumab, which spanned 2 years and compared monthly vs. as-needed administration of these drugs. Two-year results showed similar effects of these 2 medications in terms of visual gains at 2 years and losses at 5 years.3-5 The VIEW1 and VIEW2 studies compared aflibercept to ranibizumab in the treatment of nAMD, and showed non-inferiority of aflibercept to ranibizumab, and possibly suggested decreased frequency of treatment with aflibercept as compared with ranibizumab.6 The HAWK and HARRIER7 trials demonstrated noninferiority of brolucizumab to aflibercept.
While all of these medications have excellent efficacy for the treatment of nAMD, the results of HAWK and HARRIER suggests increased durability of treatment with brolucizumab, with more than 50% of patients achieving 3-month dosing of brolucizumab in HAWK and HARRIER.7
WHEN TO SWITCH?
The decision to switch anti-VEGF therapies can be a challenging one. Typically, the decision to switch therapies occurs due to either persistent fluid despite aggressive q4 week treatment, or the desire to achieve increased treatment intervals between injections. It is important to note that not all fluid is harmful, as small amounts of subretinal fluid can be safely tolerated, as shown in the FLUID study.8 However, in regards to persistent fluid, a recent study by Jaffe et al. demonstrated slightly higher efficacy of aflibercept as compared with ranibizumab for patients with persistent early retinal fluid after 3 initial monthly injections.9 Similarly, there have been additional studies showing decreased fluid and increased treatment intervals on aflibercept as compared with bevacizumab or ranibizumab.10-12 Most recently, the newly approved brolucizumab has been shown to have improved rates of fluid resolution and increased durability of treatment.7,13 However, there have been recent safety concerns with brolucizumab-related retinal vasculitis, a condition that has given pause to rapid adoption of treatment with brolucizumab.
While brolucizumab has shown promising outcomes in the treatment of nAMD, decision to treat with this medication must take the risks of retinal vasculitis into consideration.14,15
STEPWISE APPROACH
For most patients, a stepwise approach can safely be considered, starting with intravitreal bevacizumab or ranibizumab treatment and switching to a more durable treatment, such as aflibercept or brolucizumab, depending on treatment response. Each patient must have individualized treatment to their eye, with the goal of resolution of fluid and improvement of visual acuity.
While the treatment burden with the current medications is high, it is important to stress to patients the importance of compliance with therapy, and reassure them that there is the potential for new treatments on the horizon.
CASE STUDY #1
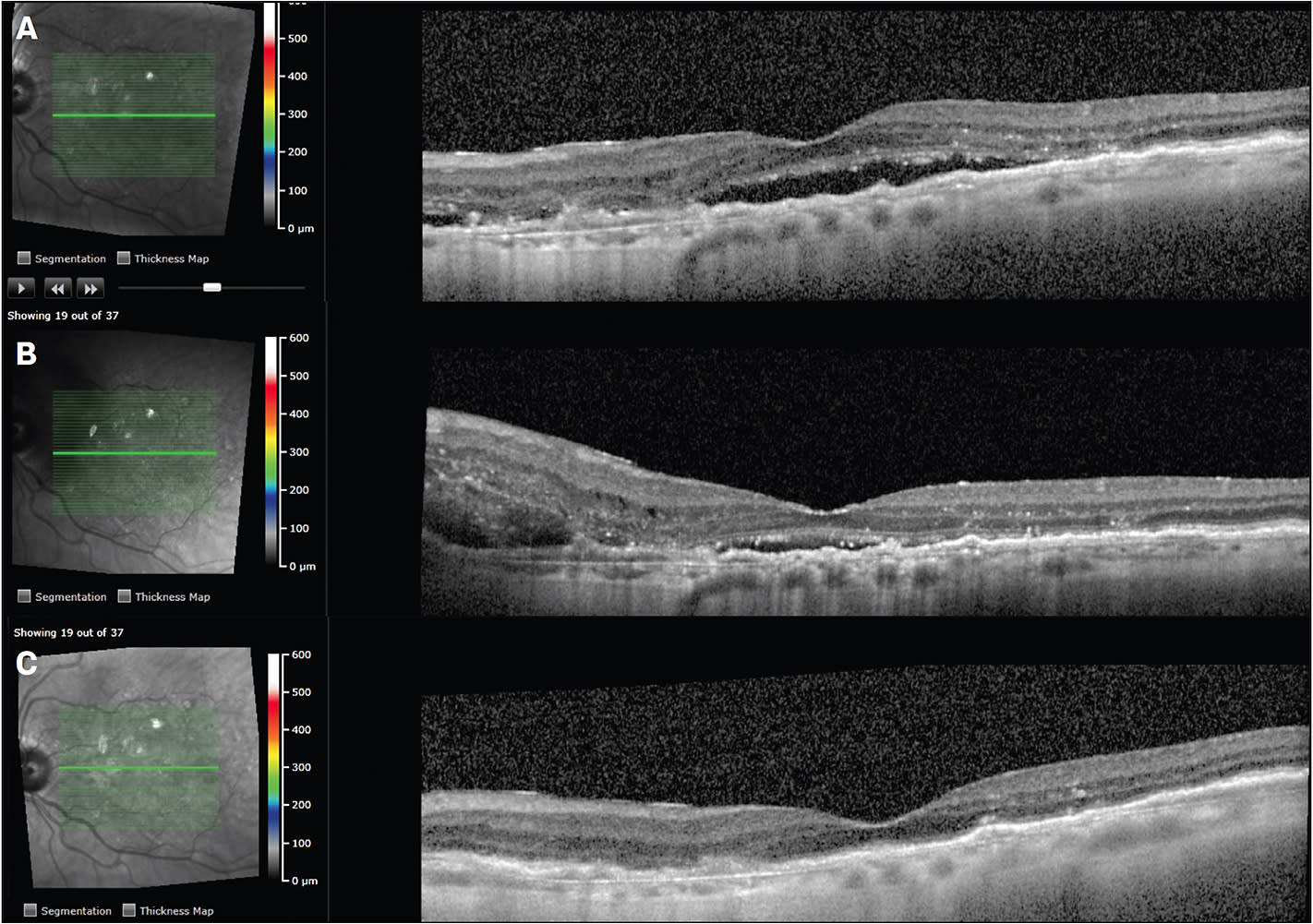
An 88-year-old female was being treated with intravitreal ranibizumab every 4 weeks for nAMD in the left eye. Visual acuity was around 20/50, and optical coherence tomography (OCT) showed persistent subretinal and intraretinal fluid despite q4 week therapy (Figure 1A). She had been treated for about 1 year on intravitreal ranibizumab, and was reluctant to switch therapies. She then presented with new subretinal peripapillary hemorrhage (Figure 1B), and the decision was made to switch to intravitreal aflibercept therapy. The patient received 6 doses of intravitreal aflibercept, treat-and-extend method, with resolution of fluid and stabilization of vision at 20/50. OCT showed resolution of subretinal hemorrhage, subretinal fluid, and intraretinal fluid (Figure 1C). She currently receives intra-vitreal aflibercept treatment around every 7 weeks in the left eye.
CASE STUDY #2
A 66-year-old male presented to the retina clinic with nAMD in the left eye (OS). Visual acuity on presentation in the left eye was counting fingers. He received intravitreal ranibizumab injections every 4 weeks for 6 total injections. Figure 2A demonstrates OCT after 6 monthly injections of ranibizumab, demonstrating persistent subretinal and intraretinal edema. Visual acuity at this time was 20/100.
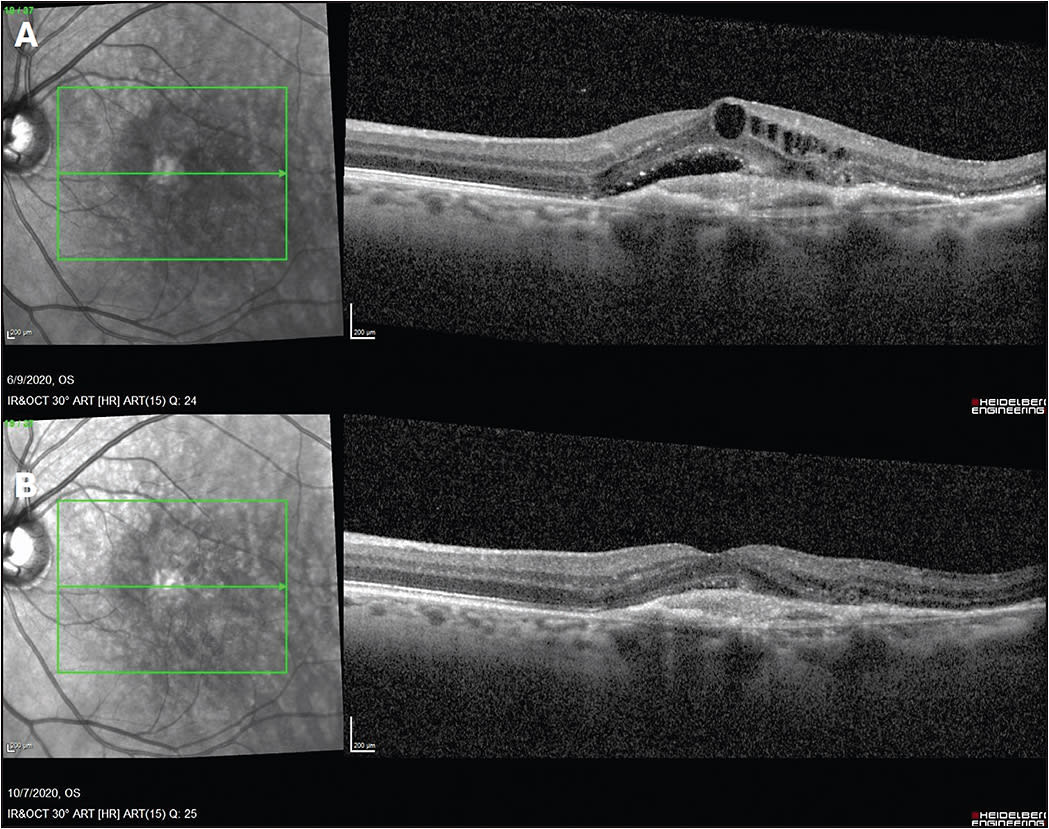
The decision was made to switch to intravitreal aflibercept. The patient received intravitreal aflibercept injections every 4 weeks for 3 injections, with significant improvement in subretinal and intraretinal fluid on OCT (Figure 2B). Visual acuity improved to 20/60. The patient has continued intravitreal aflibercept injections, now receiving them about every 5 to 6 weeks due to resolution of subretinal and intraretinal fluid. ◆
REFERENCES:
- A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration. TENAYA available at https://clinicaltrials.gov/ct2/show/NCT03823287 . Last accessed March 12, 2020. LUCERNE available at https://clinicaltrials.gov/ct2/show/NCT03823300 . Last accessed March 12, 2021.
- A phase III study to evaluate the port delivery system with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration (ARCHWAY). Available at https://clinicaltrials.gov/ct2/show/NCT03677934 . Last accessed March 12, 2021.
- Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908.
- Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398.
- Maguire MG, Martin DF, Ying GS, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123(8):1751-1761.
- Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548.
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84.
- Guymer RH, Markey CM, McAllister IL, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID Study 24-month results. Ophthalmology. 2019;126(5):723-734.
- Jaffe GJ, Kaiser PK, Thompson D, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology. 2016;123(9):1856-1864.
- Hyung C, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J of Ophthalmology. 2013;97(8):1032-1035.
- Kumar N, Marcela M, Mrejen S, et al. Voisual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33(8):1605-1612.
- Yonekawa Y, Andreoli C, Miller JB, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J of Ophthalmology. 2013;156(1):29-35.
- Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: A randomized trial. Ophthalmology. 2017;124(9):1296-1304.
- Mones J, Srivastava SK, Jaffe GJ, et al. Risks of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2020;S0161-6420(20):31075-31077.
- Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology 2020;127(10):1345-1359.








