Gene therapy aims to deliver genetic material to targeted cells, where this material can be utilized via transcription/transduction or gene editing to produce desired therapeutic proteins. An accessible, easily monitored, and immune-privileged site, the eye remains an ideal setting for gene therapy.1 The 2017 approval of voretigene neparvovec-rzyl (Luxturna; Spark Therapeutics) for biallelic RPE65 mutation-associated retinal dystrophy ushered in a new era of retinal therapeutics and holds distinction as the first gene therapy approved by the US Food and Drug Administration (FDA).2-4
Several gene therapy trials in ophthalmology have centered on monogenetic inherited retinal diseases (IRDs), including the studies leading to the approval of Luxturna. Inherited retinal diseases are attractive targets for several reasons, including precise identification of the causative gene and a more streamlined path to FDA approval for orphan diseases. An orphan disease is defined as a disease affecting <200,000 individuals, which applies to many IRDs.1,5 However, gene therapy to tackle more prevalent acquired conditions, such as age-related macular degeneration (AMD) and diabetic retinopathy, holds promise to benefit millions of patients with retinal disease.
When using gene therapy for IRDs, the goal is to create a functional protein that is normally produced by the target cell type. In the case of multifactorial, acquired conditions, gene therapy may aim to produce proteins that are not normally produced by the cell receiving genetic material. In other words, the aim is to change a cell into a “biofactory” for the production of therapeutic proteins.
GENERAL APPROACHES TO GENE THERAPY
Gene therapies differ based on the desired genetic material, the means or vehicle of delivery (typically a viral vector), and the route of administration to the target cells. How to design gene therapy will depend upon disease pathogenesis, with general approaches noted below:
- Gene augmentation: Classically used in autosomal recessive, loss-of-function IRDs, gene therapy aims to provide genetic material to produce functional, replacement protein in the targeted cell. A clear case example of this method is Luxturna for the treatment of biallelic RPE65-mediated retinal dystrophy.
- Gene inactivation: Classically used in gain-of-function IRDs, gene therapy aims to block production of an abnormal protein in the target cell (ie, the therapeutic protein produced by gene therapy may halt the production of pathogenetic, existing cellular protein). This technique may need to be coupled with gene augmentation to restore production of a functional protein.
- Gene editing: Existing DNA within the target cell is marked for removal and replacement. The technology called CRISPR (clustered regularly interspaced short palindromic repeats) is a currently available system used for gene editing, in which RNA (used to identify specific DNA) is coupled with the enzyme Cas9 to cut and remove specific DNA sequences. Although CRISPR is an exciting technology with multiple potential uses, effect of the CRISPR enzymes on unintended DNA sequences and creation of new mutations deserves caution.
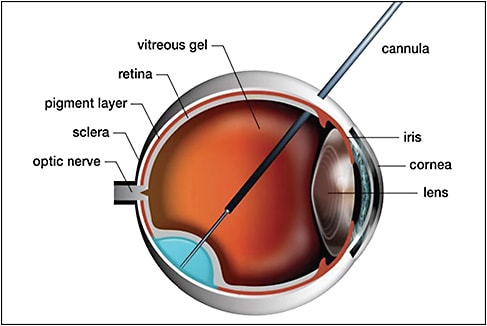
In regard to method of delivery, intravitreal injection and subretinal injection of gene therapy products have been performed. Access to the subretinal space can be achieved via a transretinal or suprachoroidal approach. When using a suprachoroidal approach, a vitrectomy can be avoided. A schematic demonstrating use of a microneedle to access the subretinal space is depicted in Figure 1, and a schematic demonstrating use of a catheter in the suprachoroidal space is depicted in Figure 2.

THE GENE THERAPY BIOFACTORY
Gene therapy for acquired, multifactorial retinal diseases will differ in many important ways from gene therapy for IRDs. Several concepts are worth considering in the design of this type of therapy:
- “Window of opportunity”: In contrast to IRDs, gene therapy for acquired retinal diseases may have a broader time interval to produce positive treatment outcomes. Moreover, candidates may not require genetic testing to confirm specific genetic defects to be candidates for therapy, as is the case for IRDs presently.
- Treatment measures: Also in contrast to trials for IRDs, gene therapy trials for acquired retinal diseases are expected to use more common outcome measures, such as best-corrected visual acuity and OCT-imaging based parameters, than trials for IRDs. For instance, in the Luxturna trials, a novel multiluminance mobility test was utilized to assess functional visual improvement.6 Additional novel functional tests may arise as more IRDs are investigated in trials.
- Steady state: In producing soluble proteins for the treatment of acquired retinal diseases — for example, production of anti-VEGF molecules for neovascular AMD — a steady state must be achieved to ensure predictable and durable treatment effect and to allow comparison to alternative treatment modalities, such as periodic intravitreal injections, and sustained-release port-delivery systems.
- Choice of target cells: While treatment of IRDs involves targeting specific cells with genetic defects, multiple cell types may be utilized as a biofactory to create soluble therapeutic proteins. This may provide the opportunity to utilize more diverse delivery vectors and routes of administration.
EARLY EFFORTS TO CREATE A BIOFACTORY FOR NEOVASCULAR AMD
Several early trials sought to utilize gene therapy for the treatment of neovascular AMD, namely the production of anti-angiogenic therapeutic proteins.7-10 These early-phase trials ended without clear efficacy to progress to phase 3 study (Table 1).
| SPONSOR | DISEASE | PHASE | VECTOR | PROTEIN | DELIVERY | SUBJECTS |
|---|---|---|---|---|---|---|
| Avalanche Technologies | Wet AMD | 1 | rAAV.sFlt-1 | VEGF receptor | Subretinal | n=8 |
| Avalanche Technologies | Wet AMD | 2a | rAAV.sFlt-1 | VEGF receptor | Subretinal | n=21 |
| Genzyme/Sanofi | Wet AMD | 1 | AAV2-sFLT01 | VEGF receptor | Intravitreal | n=19 |
| Oxford BioMedica | Wet AMD | 1 | RetinoStat | Endostatin and angiostatin | Subretinal | n=21 |
These early-stage trials were notable for a favorable safety profile, a reassuring result of several gene-therapy trials.1 Data from these trials demonstrated transgene expression and biologic activity in some patients after both intravitreal and subretinal injection of lentiviral vectors. This offers valuable early experience to design future vectors, refine therapeutic proteins of interest, and determine clinical trial endpoints.
CURRENT STUDIES
Table 2 lists gene therapy clinical trials currently indexed on clinicaltrials.gov that seek to create an ocular biofactory for the treatment of neovascular or advanced dry AMD.
| SPONSOR | DISEASE | PHASE | VECTOR | PROTEIN | DELIVERY | PLANNED SUBJECTS |
|---|---|---|---|---|---|---|
| Adverum Biotechnologies, Inc. | Wet AMD | 1 | ADVM-022 (AAV.7m8-aflibercept) | VEGF inhibitor (aflibercept) | Intravitreal | n=12 |
| Gyroscope Therapeutics | Dry AMD with geographic atrophy | 1/2 | rAAV2.CFI | Complement Factor I | Subretinal | n=35 |
| Hemera Biosciences | Wet AMD | 1 | AAVCAGsCD59 | Complement regulatory protein (sCD59) | Intravitreal | n=25 |
| Hemera Biosciences | Dry AMD with geographic atrophy | 1 | AAVCAGsCD59 | Complement regulatory protein (sCD59) | Intravitreal | n=17 |
| REGENXBIO, Inc. | Wet AMD | 1/2a | AAV8-RGX-314 | VEGF inhibitor (RGX-314, anti-VEGF Fab molecule) | Subretinal | n=42 |
CHALLENGES
Optimizing delivery vectors, desired therapeutic proteins, and route of delivery are all critical in designing effective gene therapy. The following concepts are worth special consideration:
- Capacity of vectors: The size of the desired genetic material to be transferred with gene therapy is limited by the size of the delivery vectors. As more complex retinal diseases may require both inactivation and augmentation of multiple genes, or creation of multiple soluble therapeutic proteins, multiple vector-transgene products may be required for optimal effect.
- Surgical technique: Because route of delivery of gene therapy is critical to success, reproducible surgical practices must be developed for each vector-transgene product. This may require “centers of excellence” for gene therapy to ensure adequate expertise with these techniques.
- Economic challenges: Progression to phase 3 trials for nonorphan retinal diseases will require significant capital and may limit the number of vector-transgene products tested.
CONCLUSIONS
Retinal gene therapy represents a watershed in precision medicine, with the promise of treating patients with IRDs and acquired retinal diseases alike. Use of gene therapy to turn existing cells into “biofactories” for soluble therapeutic protein production may offer a new modality for safe, durable treatment of acquired retinal diseases while minimizing treatment burden.
Continued identification of genetic defects, refinement of delivery vectors, choice in therapeutic targets, and improved surgical techniques for vector delivery will be necessary to improve outcomes. Certainly, results of existing and planned clinical trials will be of special interest to the retinal and broader medical community. RP
REFERENCES
- Bennett J. Taking stock of retinal gene therapy: looking back and moving forward. Mol Ther. 2017;25(5):1076-1094.
- Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-860.
- Maguire AM, Russell S, Wellman JA, et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126(9):1273-1285.
- FDA Approves Spark Therapeutics’ LUXTURNA (voretigene neparvovec-rzyl), a one-time gene therapy for patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy [press release]. Spark Therapeutics. December 19, 2017. ir.sparktx.com/news-releases/news-release-details/fda-approves-spark-therapeutics-luxturnatm-voretigene-neparvovec . Accessed October 8, 2019.
- Orphan Drug Act. In:1983:21 U.S.C., Ch. 29, Subch. V, Part B, §§ 360aa-360ff361, as amended.
- Chung DC, McCague S, Yu Z-F, et al. Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. Clin Experiment Ophthalmol. 2018;46(3):247-259.
- Constable IJ, Pierce CM, Lai C-M, et al. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal raav.sflt-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168-175.
- Rakoczy EP, Magno AL, Lai C-M, et al. Three-year follow-up of phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am J Ophthalmol. 2019;204:113-123.
- Heier JS, Kherani S, Desai S, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390(10089):50-61.
- Campochiaro PA, Lauer AK, Sohn EH, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2017;28(1):99-111.
Editor’s note: This article is part of a special edition of Retinal Physician that was supported by REGENXBIO.








