Retinal imaging biomarkers offer the opportunity to estimate prognosis and provide meaningful information when communicating with patients. Initial OCT biomarkers that consistently correlated with visual outcomes included integrity of the ellipsoid zone (EZ) and external limiting membrane (ELM) on spectral-domain optical coherence tomography (SD-OCT).1-3 As understanding of SD-OCT improved, a host of other imaging biomarkers were proposed. One recently described and consistently prognostic imaging biomarker has been disorganization of the retinal inner layers (DRIL). Sun et al were the first to define DRIL and demonstrate a correlation between DRIL and visual acuity (VA) in patients with diabetic macular edema (DME).4 Since then, the presence of DRIL has been reported in various retinal pathology including nonproliferative diabetic retinopathy (NPDR)5,6 and proliferative diabetic retinopathy (PDR)7 with or without DME, retinal vein occlusions (RVO),8 uveitic cystoid macular edema (CME),9 central retinal artery occlusion,10 epiretinal membranes (ERM),11,12 and macular telangiectasia.13 This article provides an overview of the studies of DRIL that highlight the importance of this OCT imaging biomarker across various disease states.
DEFINING DISORGANIZATION OF THE RETINAL INNER LAYERS
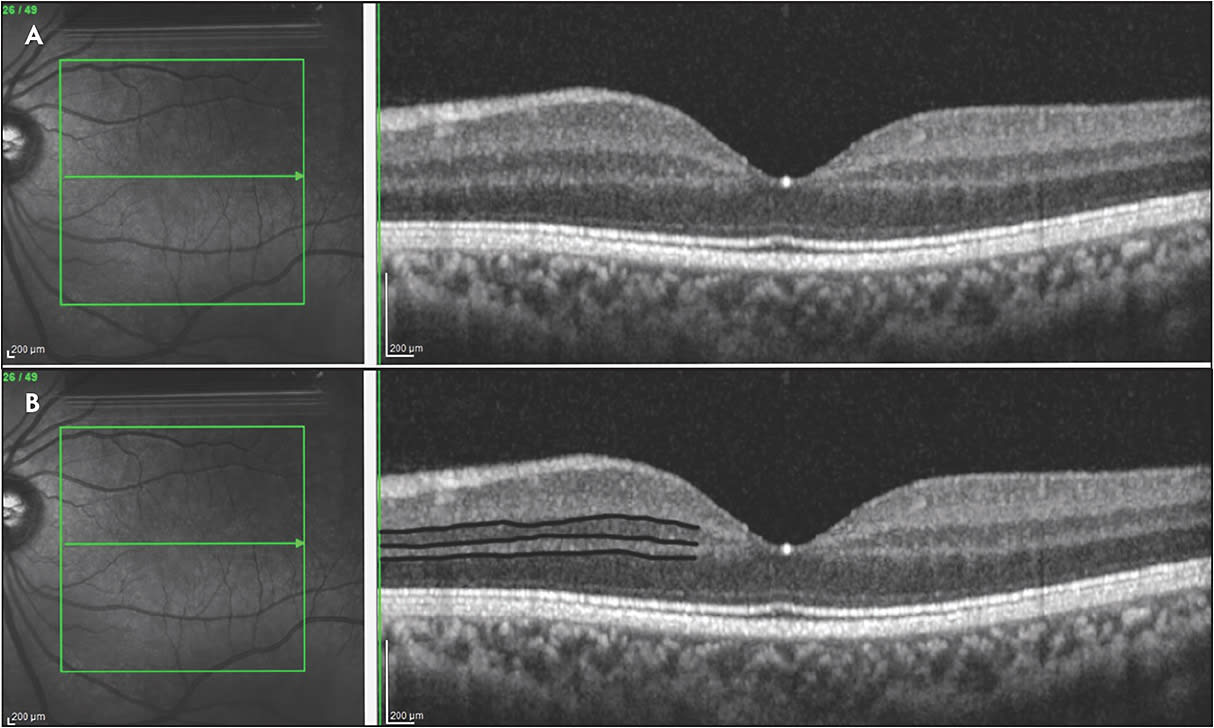
The normal retina has an orderly, laminated structure highlighted by varying reflectivity patterns on SD-OCT (Figure 1). A disorganization of these inner retinal laminations with failure to identify the boundaries between the layers is what defines DRIL. Specifically, DRIL is characterized by the inability to segment the boundaries of the inner nuclear layer (INL), outer plexiform layer (OPL), and the ganglion cell layer-inner plexiform layer (GCL-IPL) complex on OCT. The central 1,000 µm to 3,000 µm have been used by different studies as the area of interest.4,14 Various hypotheses exist for the underlying cause of DRIL. Initially it was hypothesized that DRIL may represent the disruption of the visual pathway transmission from the photoreceptors to the ganglion cells. However, histologic correlations have still not been made to support or refute this theory. More recent studies have compared areas of DRIL to OCT angiography (OCTA) imaging and found that DRIL co-associates with areas of ischemic damage that correlates to areas of absent superficial, middle, and deep capillary plexus flow.15-18 Correlation has also been made between DRIL and other OCT findings, including enlargement of the foveal avascular zone19 and disruption of the EZ and ELM.7
DIABETIC MACULAR EDEMA
Multiple diabetic retinopathy studies (with or without macular edema) have shown that a greater extent of DRIL at initial presentation correlates with worse baseline VA.4,7,20 Additionally, the extent of presenting DRIL may serve as a predictor of long-term VA following treatment.4,20,21
Interestingly, DRIL is not a fixed biomarker, and changes in DRIL over time have demonstrated prognostic value. In the initial study by Sun et al evaluating patients with DME, an increase of DRIL by 300 µm correlated to a 1-line vision decline at 8 months.4 Conversely, a decrease in DRIL by 250 µm correlated with a 1-line improvement in vision at 4 months. This finding was further supported by the work of Radwan et al, who demonstrated that both early and late resolution of DRIL in patients treated for DME corresponded to an improvement of vision compared to those with persistent DRIL.21 Figure 2 highlights a case of DRIL in a patient with chronic DME.
DIABETIC RETINOPATHY
Recent studies have suggested that DRIL may also serve as a marker for the severity of diabetic retinopathy, but larger studies are needed to validate this relationship. Joltikov et al showed that DRIL may be an important marker for early neuroretinal dysfunction prior to the appearance of other clinical findings of diabetic retinopathy.6 A recent study of 37 eyes by Nakano et al showed increased metamorphopsia with increased extent of DRIL, suggesting a broader clinical use for this imaging finding.22
RETINAL VEIN OCCLUSION
DRIL has also been studied as a marker of visual prognosis in branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO). Babiuch et al, in their study of 147 patients, found DRIL in 62% of patients. Persistence of DRIL was associated with decreased visual improvement in patients receiving anti-VEGF therapy.23 Mimouni et al similarly analyzed the relationship between DRIL and VA in 136 eyes with BRVO and CRVO. They noted that an improvement in DRIL at 4 months following 3 monthly injections was a strong predictor of final VA outcomes at 8 months. Conversely, an increase of 100 µm of DRIL correlated to half line decrease of VA.24 These findings, however, have not been uniformly replicated as subsequent studies looking at multivariate analysis to account for confounding variables have not found an association between extent of DRIL at baseline and final VA following treatment for macular edema in RVO.25,26 For instance, Berry et al did not demonstrate a correlation between baseline DRIL and final VA.27 However, following 6 months of treatment, DRIL extent on OCT was predictive of VA out to 2 years of follow-up. Interestingly, baseline ischemic index, measured as extent of nonperfusion on fluorescein angiography, was correlated with the extent of DRIL at final follow-up.27
One potential reason for the poor initial correlation and final VA involves the difficulty in quantitating DRIL at initial presentation in RVO patients with DRIL. These patients may have massive macular edema that obscures the boundaries, and it is difficult to know if this is true DRIL or cystoid spaces obliterating the boundaries of the inner retina. Babiuch et al demonstrated a low interobserver correlation in measuring DRIL supporting the difficulty in accurate measurement of DRIL in RVO patients at baseline.23 However, once the macular edema had improved after initial treatment in the Berry et al study, the correlation between DRIL and final VA became apparent.27
UVEITIC CYSTOID MACULAR EDEMA
Grewal et al evaluated the relationship between DRIL and VA in patients with uveitic CME. Foveal DRIL (DRIL >500 µm of central 1,000 µm), greater vertical extent, and greater horizontal extent of DRIL at baseline and follow-up visits were correlated with worse VA.9,14 DRIL requires excellent visualization of the retina, which is not always possible in uveitis. In 30% of patients in the series reported by Grewal et al, DRIL was not able to be assessed due to media opacities, highlighting an important limitation of this imaging biomarker in uveitic disease states.9
EPIRETINAL MEMBRANES
The prior disease states described all had a component of ischemia and inflammation contributing to the pathogenesis.28-30 In epiretinal membranes, however, the disruption of the retinal laminations is believed to be related to tangential traction acting on the retina.31,32 Various markers exist for predicting postoperative VA in ERM patients including age, duration of symptoms, preoperative VA, central macular thickness,33 and integrity of the outer retina,33,34 In the DREAM study, Zur et al looked at 90 eyes of patients with idiopathic epiretinal membranes. They characterized patients as severe, mild, or no DRIL based on borders of inner retinal layers and irregularity of the boundary. They found an average of 3-line improvement in VA in patients with mild to no DRIL compared to a 2 line or less improvement in patients with severe DRIL.12 A similar study of 46 eyes showed the presence of DRIL correlated to worse postoperative visual outcomes.11
LIMITATIONS
While DRIL may serve as a powerful VA correlate in various retinal ischemic diseases, accurate and reproducible measurements of DRIL are sometimes difficult to quantitate. In patients with macular edema secondary to RVO, there was poor interobserver correlations in DRIL.23 Macular edema may obliterate boundaries required to accurately assess DRIL. Additionally, while the underlying cause of DRIL is not known, it appears that DRIL may represent a late clinical marker of ischemia further limiting its predictive value.
CONCLUSION
A large introductory body of literature over the last few years has supported the use of this biomarker as a strong predictor of VA over time. It has correlated well with other prognostic imaging biomarkers, including EZ loss, ELM loss, and enlargement of the foveal avascular zone. Additional studies and application of this biomarker on larger data sets are warranted. Ultimately, an automated and algorithmic surrogate of DRIL is likely required prior to routine adoption of this parameter in clinical practice. In the interim, DRIL may provide the astute clinician with a valuable prognostic indicator to more accurately guide patient expectations. RP
REFERENCES
- Alasil T, Keane PA, Updike JF, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117(12):2379-2386.
- Uji A, Murakami T, Nishijima K, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012;153(4):710-717,717,e711.
- Saxena S, Srivastav K, Cheung CM, Ng JY, Lai TY. Photoreceptor inner segment ellipsoid band integrity on spectral domain optical coherence tomography. Clin Ophthalmol. 2014;8:2507-2522.
- Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316.
- Nadri G, Saxena S, Stefanickova J, et al. Disorganization of retinal inner layers correlates with ellipsoid zone disruption and retinal nerve fiber layer thinning in diabetic retinopathy. J Diabetes Complications. 2019;33(8):550-553.
- Joltikov KA, Sesi CA, de Castro VM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(13):5481-5486.
- Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202-208.
- Calugaru D, Calugaru M. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2017;184:190-191.
- Grewal DS, O’Sullivan ML, Kron M, Jaffe GJ. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol. 2017;177:116-125.
- Yilmaz H, Durukan AH. Disorganization of the retinal inner layers as a prognostic factor in eyes with central retinal artery occlusion. Int J Ophthalmol. 2019;12(6):990-995.
- Garnavou-Xirou C, Xirou T, Gkizis I, et al. The role of disorganization of retinal inner layers as predictive factor of postoperative outcome in patients with epiretinal membrane. Ophthalmic Res. 2020;63(1):13-17.
- Zur D, Iglicki M, Feldinger L, et al. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery-the DREAM study. Am J Ophthalmol. 2018;196:129-135.
- Guo J, Tang W, Ye X, et al. Predictive multi-imaging biomarkers relevant for visual acuity in idiopathic macular telangiectasis type 1. BMC Ophthalmol. 2018;18(1):69.
- Grewal DS, Hariprasad SM, Jaffe GJ. Role of disorganization of retinal inner layers as an optical coherence tomography biomarker in diabetic and uveitic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2017;48(4):282-288.
- Spaide RF. Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 2015;160(6):1200-1210.
- Onishi AC, Ashraf M, Soetikno BT, Fawzi AA. Multilevel ischemia in disorganization of the retinal inner layers on projection-resolved optical coherence tomography angiography. Retina. 2019;39(8):1588-1594.
- Nicholson L, Ramu J, Triantafyllopoulou I, et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin Exp Ophthalmol. 2015;43(8):735-741.
- Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(3):2012-2020.
- Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123(11):2352-2367.
- Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560-2570.
- Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133(7):820-825.
- Nakano E, Ota T, Jingami Y, Nakata I, Hayashi H, Yamashiro K. Correlation between metamorphopsia and disorganization of the retinal inner layers in eyes with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1873-1878.
- Babiuch AS, Han M, Conti FF, Wai K, Silva FQ, Singh RP. Association of disorganization of retinal inner layers with visual acuity response to anti-vascular endothelial growth factor therapy for macular edema secondary to retinal vein occlusion. JAMA Ophthalmol. 2019;137(1):38-46.
- Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 2017;182:160-167.
- Yiu G, Welch RJ, Wang Y, Wang Z, Wang PW, Haskova Z. Spectral-domain OCT predictors of visual outcomes after ranibizumab treatment for macular edema resulting from retinal vein occlusion. Ophthalmol Retina. 2020;4(1):67-76.
- Nakano E, Ota T, Jingami Y, Nakata I, Hayashi H, Yamashiro K. Disorganization of the retinal inner layers after anti-vegf treatment for macular edema due to branch retinal vein occlusion. Ophthalmologica. 2018;240(4):229-234.
- Berry D, Thomas AS, Fekrat S, Grewal DS. Association of disorganization of retinal inner layers with ischemic index and visual acuity in central retinal vein occlusion. Ophthalmol Retina. 2018;2(11):1125-1132.
- Usman M. An overview of our current understanding of diabetic macular ischemia (DMI). Cureus. 2018;10(7):e3064.
- Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology. 1996;103(4):551-560.
- Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159-1162.
- Oberstein SY, Byun J, Herrera D, Chapin EA, Fisher SK, Lewis GP. Cell proliferation in human epiretinal membranes: characterization of cell types and correlation with disease condition and duration. Mol Vis. 2011;17:1794-1805.
- Lee SM, Pak KY, Kwon HJ, Park SW, Lee JE, Byon IS. Association between tangential contraction and early vision loss in idiopathic epiretinal membrane. Retina. 2018;38(3):541-549.
- Sheales MP, Kingston ZS, Essex RW. Associations between preoperative OCT parameters and visual outcome 3 months postoperatively in patients undergoing vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1909-1917.
- Hosoda Y, Ooto S, Hangai M, Oishi A, Yoshimura N. Foveal photoreceptor deformation as a significant predictor of postoperative visual outcome in idiopathic epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2015;56(11):6387-6393.








