Microincisional vitrectomy surgery instrumentation has changed dramatically since the early 1970s when Dr. Robert Machemer performed the first vitrectomy. Smaller gauges and superior technology have enabled surgeons to provide exceptional outcomes when complex vitreoretinal pathology is present. Currently, there is an international trend toward combined phacoemulsification and vitrectomy due to several potential benefits including faster visual recovery, reduced costs, improved patient convenience, and reduced rates of retained lens fragments compared to sequential surgeries.1-4 The literature regarding the therapeutic benefits of combined phacovitrectomy is extensive in a wide array of pathologies, including complex retinal detachments, posterior-segment tumors, complicated cataracts, trauma, uveitis, epiretinal membranes, and vitreous opacities.5-7 Phacoemulsification following vitrectomy also carries additional risks including posterior capsular tears, zonular dialysis and loss of lens particles posteriorly.8 This article will describe clinical pearls to perform successful microincisional phacovitrectomy and address ongoing challenges of combined vs sequential surgery.
PATIENT SELECTION
The key to successful phacovitrectomy is appropriate patient selection. Patients with visually significant cataracts and coexistent or suspected surgical posterior segment pathology benefit the most from combined microincisional phacovitrectomy. However, patients with early lenticular changes who would likely have visual loss due to cataract progression postoperatively are also excellent candidates. Delaying cataract surgery in patients with peripheral pathology or suboptimal optical axis may lessen the surgical outcomes because surgical success is fundamentally associated to visualization and maneuverability capabilities.
Pediatric patients in the amblyogenic age range with a clear crystalline lens and excellent visual potential may benefit from sequential surgery, because preservation of accommodation facilitates amblyopia therapy. However, certain pediatric patients with a clear crystalline lens may also benefit from combined phacovitrectomy and lens implantation, especially those with significant comorbidities such as retinal detachment with far peripheral pathology, proliferative vitreoretinopathy, high refractive errors, anisometropia, and unilateral amblyopia.9,10
In general, intraocular lens implantation should be avoided in children with a corneal diameter less than 9 mm.9 Family members need to be aware that a second surgery will be warranted in the near future.
Complex vitreoretinal pathology should be individualized. Patients with pre-existing lenticular changes who will undergo macular surgery may benefit significantly from combined phacovitrectomy because it allows optimization of the visual axis and larger margin for visual acuity improvement. Patients with active uveitis, macula-detached retinal detachments, anterior proliferative vitreoretinopathy, and/or recurrent retinal detachments may benefit from lens extraction and aphakia at the time of primary vitrectomy. In contrast, patients with macula-attached retinal detachments and quiescent uveitic eyes may benefit from phacovitrectomy with intraocular lens implantation at the time of initial repair to avoid a second surgery and improve peripheral visualization at the time of primary repair. On the other hand, eyes undergoing scleral buckling at the time of vitrectomy should undergo sequential cataract surgery to achieve accurate postoperative refractive outcomes. Preoperative, intraoperative, and postoperative pharmacotherapy should be considered in eyes susceptible to a vigorous postsurgical inflammatory reaction. To achieve optimal outcomes, a comprehensive assessment of the pathology must be performed and anticipation of future steps in management should be considered.
KEY SURGICAL PEARLS
The ability to perform effective combination of phacoemulsification with vitrectomy depends on understanding the significance of each step and the possible variations that may be performed. Although similarities with routine small-incision cataract surgery are recognizable, to approach the posterior segment, certain variations in technique should be considered.
Presurgical Evaluation
A complete ophthalmologic evaluation prior to surgery is vital prior to devising a surgical plan. Attention to the external anatomy, including anterior surface, restrictive processes, glaucoma shunts, and scleral buckles, is important because it may affect the surgical approach. Patients using alpha-1 adrenergic receptor antagonists and other agents associated with floppy iris syndrome should be cautioned of potential complications. The availability of iris hooks and other tools to manually enlarge the pupil should always be available when posterior synechiae and iris neovascular membranes (eg, neovascular glaucoma) are present. Patients with complex medical history and/or pathology may benefit from having multiple tools readily accessible in the operating room.
Refraction and biometry should be carefully evaluated prior to surgery. Patients with significant astigmatism may benefit from individualized placement of incisions. Multifocal and toric intraocular lenses may be placed in carefully selected patients. A thorough discussion regarding refractive outcomes should be undertaken prior to surgery to minimize unrealistic expectations.
Sclerotomies
Many vitreoretinal surgeons approach surgery from the superior aspect and in this circumstance, the sclerotomies should be placed near the horizontal meridian to maximize ocular mobility, reduce eyelid touch, and facilitate easier access to the superior aspect of the retina. However, sclerotomy location should be individualized to each patient. Sclerotomies should avoid filtering blebs, ocular tumors, tube shunts, and prior corneal or scleral grafts. The placement should optimize the angle for intraocular access and comfort. Adequate spacing of the cannulas also allows correct orientation of the corneal incision and minimal obstruction during the phacoemulsification portion of the surgery.
Valved Trochars
A basic pearl for phacovitrectomy is to place the trochar-cannula system at the beginning of surgery, even if cataract surgery is going to be performed first (Figure 1). Placement of the trochars after cataract surgery may result in opening of the corneal incisions and intraocular lens subluxation at the time of placement. The infusion cannula should be connected but turned off until the anterior part of the procedure has concluded to decrease posterior pressure and shallowing of the anterior segment. Early trochar placement also permits the surgeon to decompress the posterior segment if the anterior chamber becomes uncomfortably shallow for safe manipulation such as in pediatric or nanophthalmic eyes. If a posterior capsular tear is present during surgery, pre-placed trochars allow quick access to the posterior segment.
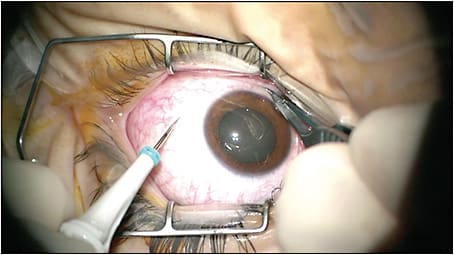
The introduction of valved trochars has enhanced the fluidics of combined phacovitrectomy by preventing aqueous reflux through an open cannula and stabilizing intraocular pressure during surgery. This may lower the risks of intraoperative hypotony, suprachoroidal hemorrhage, choroidal detachment, and globe deformation during surgery. Difficulty introducing instruments such as the soft-tipped extrusion cannula through the valved cannula may be experienced. However, the stabilization of the fluid dynamics during surgery outweighs any difficulties especially when complex pathology is present.
Corneal Incisions
Corneal incisions should be placed to optimize the surgical approach of the lens while considering existing trochar placement (Figure 2). Choosing the location of the corneal incisions at the steep axis of corneal astigmatism improves astigmatic postoperative correction. Older adults typically have against-the-rule astigmatism and benefit from placement of incisions in the horizontal meridian. We routinely suture the main corneal incision prior to vitrectomy to decrease the possibility of iris prolapse and anterior segment instability, especially if high intraocular pressure may be needed. Completely filling the anterior segment with viscoelastic prior to vitrectomy increases pupil dilation while minimizing iris prolapse, lens displacement, and anterior migration of air/gas or silicone oil during and immediately following surgery.

Incorporation of the femtosecond laser into the construction of corneal incisions may aid surgeons in providing unprecedented refractive outcomes when performing phacovitrectomy. A limbal-relaxing incision may also be performed in carefully selected patients.11,12
Capsulorrhexis
The success of phacovitrectomy is largely dependent on the continuous curvilinear capsulorrhexis. If the red reflex is limited due to vitreous opacity or hemorrhage, retroillumination with the endoilluminator inserted through the pars plana cannula can help improve contrast of the anterior capsule. Alternatively, trypan blue may be used to stain the anterior capsule after air infusion into the anterior segment (Figure 3). In adults, we perform a large diameter capsulorrhexis of approximately 6.5 mm in diameter to easily manipulate the nucleus during phacoemulsification and to maximize the peripheral view postoperatively. Alternatively, performing a smaller capsulorrhexis may limit lens displacement if gas tamponade is needed.
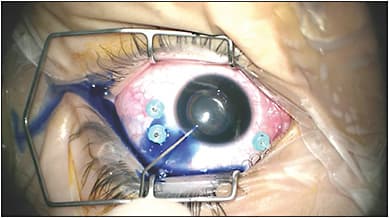
If a toric or multifocal intraocular lens is planned, a smaller diameter capsulorrhexis may be preferred. Femtosecond laser assisted capsulorrhexis and fragmentation may be considered in select cases of combined phacovitrectomy to perform a smaller diameter capsulorrhexis without the difficulty associated to limited maneuverability during phacoemulsification.11-14 Still, case series of femtosecond laser assisted phacovitrectomy are limited and long-term follow-up data are unavailable.
Phacoemulsification
We routinely perform phacoemulsification prior to vitrectomy to optimize the view of the posterior segment and facilitate peripheral dissection during subsequent vitrectomy. This order also minimizes posterior capsule instability during phacoemulsification. However, in cases of a dense vitreous hemorrhage, clearing the opacity from the posterior segment to elicit a red reflex may be beneficial prior to phacoemulsification.
Pediatric patients do not need to undergo phacoemulsification of the nucleus because the crystalline lens is soft. We routinely use the vitrector in aspiration mode using an anterior segment approach to remove the lens nucleus and the cortical material. Other variations of this technique include using the irrigation-aspiration handpiece, Simcoe cannula, or phacoemulsification handpiece in aspiration mode.
Intraocular Lenses
Intraocular lens implantation at the time of primary combined cataract and vitrectomy surgery depends on many factors. Pediatric patients younger that 1 year of age may not be good candidates for standard intraocular lens implantation due to inadequately small anterior segment. Patients with anterior traction or anterior proliferative vitreoretinopathy, endophthalmitis, peripheral tumors, trauma, and uveitis may also benefit from aphakia and secondary lens implantation.
Studies have also reported the outcomes of combined cataract surgery with toric intraocular lens implantation when performed in conjunction with transconjunctival sutureless pars plana vitrectomy.15-16 These studies suggest that toric lens position and axis remained stable after implantation during combined cataract small gauge vitrectomy surgery with and without gas tamponade.
Currently, we prefer to use a 3-piece intraocular lens due to increased stability in the sulcus and the capsular bag (Figure 4). However, the choice of intraocular lens varies depending on surgeon expertise and lens availability. No prospective studies to date have evaluated the outcomes of multifocal or accommodative intraocular lens implants in patients undergoing combined cataract extraction and vitrectomy surgery.

OUTCOMES OF COMBINED VS SEQUENTIAL SURGERY
A recent prospective study evaluated 120 patients who underwent 25-gauge phacovitrectomy without gas injection for macular pathology against patients who underwent phacoemulsification alone for cataract.1 There was no significant difference in the refractive error between both groups at 3 days, 1 month, and 3 months after surgery. The study concluded that there were no refractive shifts or intraocular lens displacements associated to phacovitrectomy when performed without tamponade.
Refractive outcomes of patients undergoing phacovitrectomy with intraocular lens implantation vs cataract surgery in previously vitrectomized eyes have also been evaluated.2 A study that included 55 eyes that underwent phacovitrectomy for epiretinal membranes or macular holes and 54 eyes that underwent cataract surgery alone following vitrectomy found a mean biometry prediction error of 0.59 D and 0.35 D, respectively. Mean refractive error was significantly less in the group that underwent sequential surgery; however, hyperopic postoperative errors were more than twice as likely in eyes that underwent sequential surgery. Overall, the refractive differences among the groups may not be clinically significant. Similarly, studies that have evaluated the refractive outcomes after vitrectomy did not find any significant change in refraction after vitrectomy in pseudophakic patients.17
A recent study evaluated 266 eyes that underwent either combined phacovitrectomy or vitrectomy alone for primary retinal detachment.3 The primary anatomical success rate was 84.3% in the combined group and 89.2% in the vitrectomy group (P=.311). Most eyes (78.4%) that underwent vitrectomy alone required cataract extraction during the follow-up period. There was no significant difference between groups in terms of the mean final best-corrected visual acuity (P=.185). The study concluded that combined surgery was safe and effective for retinal detachment surgery with comparable functional results.
COMPLICATIONS
The most common complication following phacovitrectomy is posterior capsule opacity (~10%) formation following surgery.18 YAG laser can be performed 3 months after surgery or at the time of primary phacovitrectomy (Figure 5). Other less common complications include posterior synechiae (4.2%), uveitis (2.1%), angle closure glaucoma (1.6%), and rhegmatogenous retinal detachment (1.1%).

CONCLUSION
Improvements in microincisional surgery during the last decade have allowed improved outcomes for combined phacovitrecomy. The ability to perform small incisions in both the anterior and posterior segment has enhanced fluidic and intraocular pressure control. Retinal detachment rates are comparable to cataract surgery alone in cases with high-risk characteristics (Figure 6). Phacovitrectomy minimizes both the time and expense of visual recovery and the need for additional surgery. We expect this technique to continue to expand due to continual technological improvements that may improve safety and efficacy while ultimately improving anatomic and visual outcomes in these complex cases. RP

REFERENCES
- Sato T, Korehisa H, Shibata S, Hayashi K. Prospective comparison of intraocular lens dynamics and refractive error between phacovitrectomy and phacoemulsification alone. Ophthalmol Retina. February 4, 2020. [Online ahead of print]
- Tranos PG, Allan B, Balidis M, et al. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):987-993.
- Tan A, Bertrand-Boiché M, Angioi-Duprez K, Berrod JP, Conart JB. Outcomes of combined phacoemulsification and pars plana vitrectomy for rhegmatogenous retinal detachment: a comparative study. Retina. April 3, 2020. [Online ahead of print]
- Hertzberg SNW, Veiby NCBB, Bragadottir R, et al. Cost-effectiveness of the triple procedure - phacovitrectomy with posterior capsulotomy compared to phacovitrectomy and sequential procedures. Acta Ophthalmol. February 20, 2020. [Online ahead of print]
- Czajka MP, Frajdenberg A, Johansson B. Outcomes after combined 1.8-mm microincision cataract surgery and 23-gauge transconjunctival vitrectomy for posterior segment disease: a retrospective study. Retina. 2014;34(1):142-148.
- Parke DW 3rd, Sisk RA, Murray TG. Intraoperative intravitreal triamcinolone decreases macular edema after vitrectomy with phacoemulsification. Clin Ophthalmol. 2012;6:1347-1353.
- Sisk RA, Murray TG. Combined phacoemulsification and sutureless 23-gauge pars plana vitrectomy for complex vitreoretinal diseases. Br J Ophthalmol. 2010;94(8):1028-1032.
- McDermott ML, Puklin JE, Abrams GW. Phacoemulsification for cataract following pars plana vitrectomy. Ophthalmic Surg Lasers. 1997;28:558-569.
- Yu YS, Lee JH, Chang BL. Surgical management of congenital cataract associated with severe microphthalmos. J Cataract Refract Surg. 2000;26(8):1219-1224.
- Albavera-Giles T, Serna-Ojeda JC, Jimenez-Corona A, Pedroza-Seres M. Outcomes of cataract surgery with/without vitrectomy in patients with pars planitis and immunosuppressive therapy. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1213-1219.
- Moya Romero JO, Ochoa Máynez GA, Cantero Vergara MA, Gómez Cortes CA. Femtophacovitrectomy. Case series and description of the technique. Arch Soc Esp Oftalmol. 2016 Oct;91(10):461-468.
- Bali SJ, Hodge C, Chen S, Sutton G. Femtosecond laser assisted cataract surgery in phacovitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1549-1551.
- Kelkar A, Kelkar J, Chitale S, Shah R, Jain A, Kelkar S. To assess surgical outcomes of combined femtosecond laser-assisted cataract surgery with 25-gauge vitrectomy surgery at a tertiary eye care center. Indian J Ophthalmol. 2016;64(8):584-588.
- Dollin M, Garg SJ, Pendse S. Pars plana lensectomy after femtosecond laser-assisted cataract surgery. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):680-682.
- Kolozsvári BL, Losonczy G, Pásztor D, Fodor M. Correction of irregular and induced regular corneal astigmatism with toric IOL after posterior segment surgery: a case series. BMC Ophthalmol. 2017;17(1):3.
- Toussaint BW, Appenzeller MF, Miller DM, et al. Stability of the Acrysof toric intraocular lens in combined cataract surgery and transconjunctival sutureless vitrectomy. Retina. 2015;35(6):1065-1071.
- Emanuelli A, García-Gonzalez JM, Berrocal MH, Flynn HW Jr. Minimal refractive change induced by sutureless 23- and 25-gauge pars plana vitrectomy. Ophthalmic Surg Lasers Imaging. 2012;43(2):94-96.
- Fajgenbaum MAP, Neffendorf JE, Wong RS, Laidlaw DAH, Williamson TH. Intraoperative and postoperative complications in phacovitrectomy for epiretinal membrane and macular hole: a clinical audit of 1,000 consecutive eyes. Retina. 2018;38(9):1865-1872.








