Macular edema (ME) related to branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO; Figures 1 and 2) is a common cause of vision loss in the industrialized world. According to the 2017 American Society of Retina Specialists Preferences and Trends survey, more than 90% of US and international retina specialists use anti-vascular endothelial growth factor (anti-VEGF) medications as an initial treatment of choice for these RVO patients with moderate vision loss due to ME.1 In randomized clinical trials (RCTs), 3 anti-VEGF agents, ranibizumab (Lucentis; Genentech), aflibercept (Eylea; Regeneron Pharmaceuticals), and off-label bevacizumab (Avastin; Genentech), have achieved dramatic results in treatment of these patients.
As the health care system shifts from volume-based fee-for-service systems to value-based systems, real-world outcomes assume progressively greater importance, especially to benchmark therapies and healthcare systems for quality measures and reimbursements. However, there have been few large analyses of clinical outcomes of anti-VEGF therapy for RVO-related ME. Recently, my colleagues and I assessed these clinical outcomes from a large database of aggregated, longitudinal EMRs from a geographically and demographically diverse sample of US retina specialists (the Vestrum Health retina research database), using a similar approach to our prior studies in other disorders.2-5 Treatment-naïve RVO patients with ME who underwent anti-VEGF injections between 2013 and 2019 were eligible, if follow-up data were available through 12 months.
This analysis included 12,214 patient eyes with ME related to RVO. In those 6,914 eyes with BRVO-related ME, mean age at presentation was 72.3 years and 56% were female. Mean baseline VA was 56.6 letters (20/80 Snellen equivalent). In those 5,300 eyes with CRVO-related ME, the mean age at presentation was 72.9 years, and 51% were female. Mean baseline visual acuity (VA) was 39.5 letters (20/160 Snellen equivalent). While all received anti-VEGF therapy, a minority of patient eyes also received intravitreal corticosteroids (9% BRVO, 9% CRVO), focal laser (12% BRVO, 2% CRVO), and panretinal laser (3% BRVO, 6% CRVO), reflecting the real-world nature of this analysis.
REAL-WORLD OUTCOMES
Mining EMR data has numerous limitations, including its retrospective nature and the utilization of aggregated data, as well as nonstandardized VA assessment from multiple sites. Nevertheless, the resulting data can yield important longitudinal insights to better understand patient outcomes in clinical practice and to provide context for value-based health care systems. Overall, our US-based study demonstrated fewer anti-VEGF injections and worse visual outcomes by approximately 2 lines of vision (10 letters), compared to patients receiving therapy in RCTs (Figure 3).
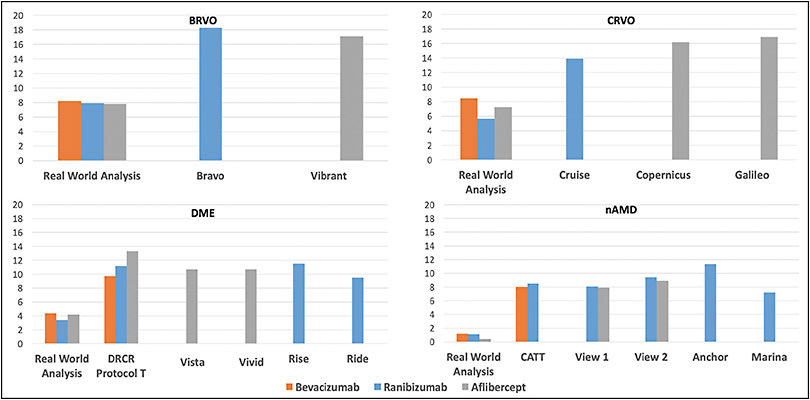
Specifically, for BRVO-related ME, at 1 year, after a mean of 7.4 anti-VEGF injections, there was a mean gain of 8.1 letters (95% confidence interval [CI] for change in VA, +7.55 to +8.57; P<.001). In contrast, the BRAVO registration trial for ranibizumab demonstrated a best-corrected visual acuity (BVCA) improvement of 18.3 letters at 1 year, compared to baseline.6,7 In BRAVO, patients received monthly treatment for the first 6 months, followed by as-needed treatment for the second 6 months (mean 2.7 injections).7 Interestingly, although 12% of the real-world patient eyes received focal laser treatment over 1 year, an even larger 19.8% of ranibizumab-treated patient eyes in BRAVO also received focal laser in the first 6 months.6 For aflibercept, the VIBRANT registration trial demonstrated a similar improvement to BRAVO, with a 17.1 letter gain at 12 months, with patients receiving monthly treatment for the first 6 months, followed by bimonthly treatment for the second 6 months.8,9
For CRVO-related ME, at 1 year, after a mean of 7.6 anti-VEGF injections, our real-world analysis revealed a mean gain of 7.1 letters (95% CI for change in VA, +6.31 to +7.95; P<.001). In contrast, the CRUISE registration trial for ranibizumab demonstrated a BVCA improvement of 13.9 letters at 1 year, compared to baseline.10,11 In CRUISE, patients received monthly treatment for the first 6 months, followed by as-needed treatment for the second 6 months (mean 3.3 injections). In 2 registration trials for aflibercept, COPERNICUS and GALILEO, BVCA improved by 16.2 letters and 16.9 letters at 1 year, respectively, compared to baseline.12,13 Patients received monthly treatment for the first 6 months, followed by as-needed treatment for the second 6 months (mean 2.7 and 2.5 injections, respectively). For a bevacizumab RCT comparison, the SCORE2 trial in CRVO-related ME demonstrated similar 6-month BCVA improvements for monthly aflibercept and bevacizumab.14
Cross-trial comparisons have limitations, including nonstandardized VA assessments in real-world studies. For example, our patients were slightly older than those patients in the aforementioned RCTs, with similar baseline VA for BRVO patients, but with worse baseline VA for CRVO patients; some patients also received additional therapies, but some of the RCTs also included laser rescue. In addition, real-world studies are prone to worse therapeutic outcomes, given the more diverse patient presentations, likely including advanced disease states such as ischemia. Nevertheless, a strength of this real-world study includes the large sample size, and although the exact magnitude of the differences in visual gain vs RCTs may be debatable, there is likely a meaningful shortfall, which highlights unmet need for more effective and longer duration therapies to address treatment burden.
VISUAL OUTCOMES RELATIVE TO TREATMENT INTENSITY
Although RVO patients in the aforementioned RCTs received 6 fixed monthly treatments in the first 6 months, 25% of real-world BRVO patients and 22% of CRVO patients received fewer treatments over 1 year. When stratified by anti-VEGF injection frequency, VA gain at 1 year increased with increasing injection frequency on average, which is expected (Figure 4). For both BRVO-related ME and CRVO-related ME, there was generally a linear relationship between mean letters gained and mean number of anti-VEGF injections at 1 year, with a mean gain of nearly 1 letter for each addition anti-VEGF injection. At the lower range, those patient eyes that underwent ≤5 injections in 1 year generally improved minimally, gaining less than 1 line of vision (<5 letters). Those who received ≥9 injections in 1 year generally gained more than 2 lines of vision (>10 letters).
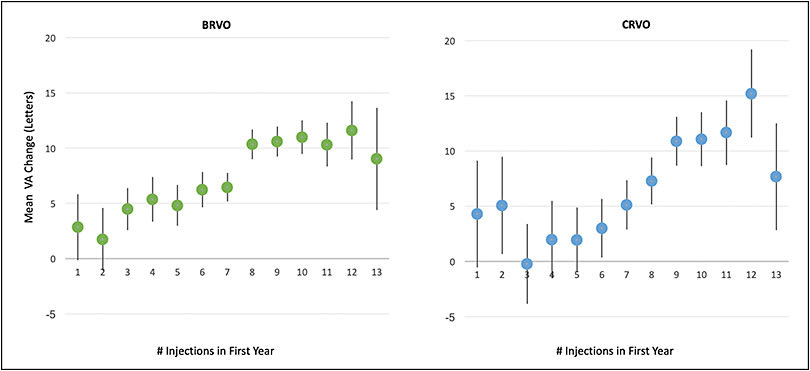
When stratified by both baseline VA and treatment intensity, the trends persisted. Overall, the mean 1-year VA change improved with both increased anti-VEGF injection frequency and decreased baseline VA. For example, in both BRVO-related ME and CRVO-related ME, patients with good baseline VA of 20/40 or better generally lost vision, reflecting a ceiling effect. In contrast, those patient eyes with moderately severe VA loss at presentation (baseline VA between 20/70 and 20/200) who received ≥9 injections in 1 year generally improved by approximately 3 or more lines (≥15 letters) for BRVO-related ME and 2 or more lines (≥10 letters) for CRVO-related ME, which reassuringly demonstrates that more intense therapy with current anti-VEGF agents yields meaningful benefit in clinical practice.
ONE-YEAR VISION GAINS IN RVO
Although cross-trial comparisons have limitations, they can provide clinical context across common retinal disorders treated with the same anti-VEGF agents. Notably, the real-world 1-year VA gain for RVO-related ME compares favorably to the real-world 1-year VA gain for neovascular AMD (nAMD) and DME, using the same database (Figure 3). Specifically, real-world BRVO-related ME patients gain 8.1 letters after an average of 7.4 injections at 1 year, and CRVO-related ME patients gain 7.1 letters after an average of 7.6 injections at 1 year, while our recent study with corresponding eligibility criteria in 49,485 nAMD patient eyes demonstrated only a 1.0-letter gain after an average of 7.3 anti-VEGF injections.4 Likewise, our recent study with corresponding eligibility criteria in 28,658 DME patient eyes showed a mean gain of 4.2 letters after an average of 6.4 anti-VEGF injections at 1 year.5
However, although real-world RVO-related ME patients experience greater 1-year visual gains than real-world patients with AMD and DME, they exhibit a larger gap in visual gain when compared to respective RCTs (Figure 3). Specifically, for BRVO-related ME, the registration trials highlighted above show an average 1-year BCVA improvement of 17.7 letters, and for CRVO-related ME, RCTs show an improvement of 15.7 letters.7,8,10,12,13 For nAMD, registration trials for ranibizumab and aflibercept as well as the monthly ranibizumab and bevacizumab arms of the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) suggested that these anti-VEGF agents perform similarly, yielding an average 1-year BCVA improvement of 8.7 letters.15-18 Likewise, for DME, registration trials for ranibizumab and aflibercept, as well as the DRCR Retina Network comparative effectiveness trial (Protocol T), demonstrate an average 1-year BCVA improvement of 10.9 letters with these anti-VEGF agents.19-21 Finally, real-world studies also demonstrate that RVO, nAMD, and DME patients in clinical practice are consistently undertreated compared to RCTs, highlighting the treatment burden with current therapeutic regimens.
THE FUTURE
Patients with RVO-related ME in the United States exhibit meaningfully worse 1-year VA gains than patients in RCTs. The mean 1-year VA change tended to improve in patient eyes with both increased anti-VEGF injection frequency and decreased baseline VA, although there were ceiling effects related to baseline VA. Also, this study demonstrates that real-world RVO-related ME patient eyes with relatively good baseline VA are generally at risk of VA loss at 1 year. In contrast, those patient eyes with moderately severe VA loss at presentation (baseline VA between 20/70 and 20/200) who received ≥9 injections generally improved by approximately 3 lines (≥15 letters) for BRVO-related ME and 2 lines (≥10 letters) for CRVO-related ME, which demonstrates that more intense therapy with current anti-VEGF agents yields meaningful benefit in clinical practice. These real-world outcomes for RVO-related ME compare favorably to the 1-year real-world outcomes for anti-VEGF therapy in nAMD and DME, using the same database, but real-world RVO-related ME patients exhibit a larger gap in visual gain when compared to respective RCTs. Overall, as the health care system shifts to value-based approaches, these clinical outcome studies assume greater importance, especially for benchmarking. In addition, these results highlight the necessity for patient counselling on treatment frequency with current anti-VEGF therapy and also highlight the unmet need for more effective and longer duration therapies to address treatment burden. RP
REFERENCES
- Shah GK. ASRS 2017 preferences and trends membership survey. Chicago: American Society of Retina Specialists.
- Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2(12):1179-1187.
- Ciulla TA, Huang F, Westby K, et al. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2018;2(7):645-653.
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4(1):19-30.
- Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2020. [Online ahead of print]
- Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.
- Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594-602.
- Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123(2):330-336.
- Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122(3):538-544.
- Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041-2049.
- Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.
- Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155(3):429-437.
- Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology. 2014;121(1):202-208.
- Scott IU, Vanveldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444.
- CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908.
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254.
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801.
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203.








