Retina specialists use and analyze optical coherence tomography (OCT) images daily for most patients.1 Development of spectral-domain OCT (SD-OCT) provided improvement in resolution and speed of acquisition, which in turn allowed for more detailed visualization of vitreoretinal pathology.2 Optical coherence tomography has been an invaluable clinical tool aiding in pre-operative diagnosis and postoperative monitoring of many surgical retinal diseases such as epiretinal membranes (ERM), macular holes (MH), rhegmatogenous retinal detachment (RRD), and tractional retinal detachment (TRD).3-8 Recently, intraoperative OCT (iOCT) has emerged as a useful tool by bringing real-time OCT scanning and all of its functional utility to the operating room during vitreoretinal surgery.9-11
HANDHELD iOCT
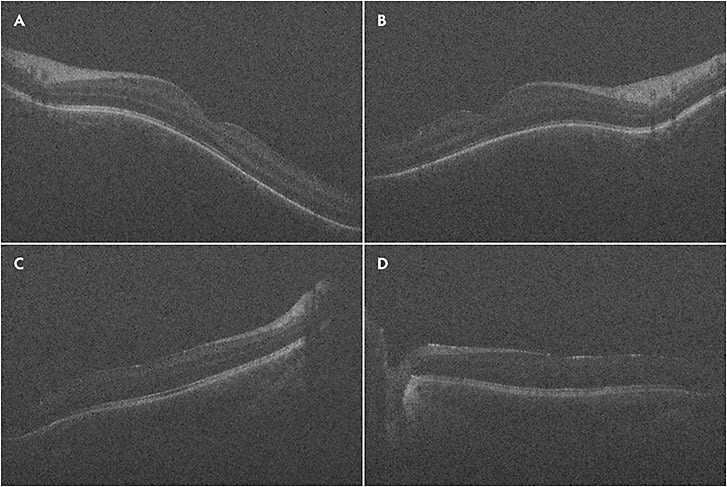
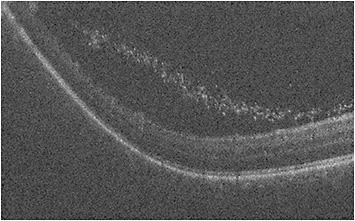
The advent of handheld OCT probes made imaging supine patients during retinal surgery feasible. In 2009, Toth et al first reported using a handheld SD-iOCT system on patients undergoing macular surgery for diseases such as ERM, MH, vitreomacular traction, and retinopathy of prematurity (ROP).12-14 The FDA-approved portable iOCT systems are the Envisu handheld OCT probe (Leica Microsystems) and the stand-mounted iVue system (Optovue). Since the publication of the report by Toth et al, several others have reported using handheld iOCT for other indications, such as exam under anesthesia (EUA) for pediatric patients with albinism, ROP, and shaken baby syndrome,15-17 as well as for surgical indications such as RD,18 MH,19,20 and other macular indications.21 At our institution, we routinely use the Envisu handheld iOCT probe for pediatric EUAs. For example, we use the handheld OCT to assess and document the foveal structural appearance in cases of EUA for ROP (Figure 1). Additionally, the handheld OCT can characterize peripheral pathology such as extravascular fibrous proliferation (EFP) or “popcorn” lesions, which can be subtle on examination alone (Figure 2).
MICROSCOPE-MOUNTED iOCT
When using a portable handheld iOCT probe, surgeons need to pause surgical procedures to acquire images, increasing intraoperative time and risk of contaminating the surgical field. Microscope-mounted iOCT devices have facilitated more image stability. Additional benefits include a decrease in image capture time, improvement of reproducibility, and ease of use with microscope foot pedal control.22-24 Although microscope-mounted iOCT allowed for easier alignment of the system, real-time visualization of the tissue and concurrent tissue–instrument interactions was not possible until the advent of microscope-integrated intraoperative OCT (Mi-iOCT) devices.25,26
MICROSCOPE-INTEGRATED iOCT
Microscope-integrated iOCT systems incorporate the OCT optical path into the common optical pathway of the surgical microscope, allowing improved targeting and tracking of the scan beam and achieving parfocal and coaxial OCT imaging with the surgical view. With Mi-iOCT, the surgeon has real-time visualization and feedback while performing surgical maneuvers.
The first publication of Mi-iOCT use in vitreoretinal surgery was by Toth in 2010, describing a custom prototype system with a research OCT that was integrated with a commercially available operating microscope.27 Other early prototypes included the Cirrus SD-OCT (Carl Zeiss Meditec) using the Zeiss OPMI VISU 200 surgical microscope,23 and Enfocus (Leica Microsystems).28
Currently, 3 systems are approved by the US Food and Drug Administration. The first to be approved was the Zeiss RESCAN 700, which is built on the Zeiss Lumera 700 microscope platform.29-31 The RESCAN 700 has Z-axis tracking and the iOCT data are provided through the surgeon’s oculars. Control of the OCT system can be performed via the foot pedals. Second to be approved was the Haag-Streit Surgical iOCT system, which is integrated through a microscope side port and uses the OPMedT OCT system including both microscope-mounted and heads-up display screens.32 Third is the Enfocus system, which can be configured with Leica surgical microscopes and uses an external monitor for display.9,28
CLINICAL TRIALS
Early studies evaluated the feasibility of iOCT during vitreoretinal surgery, but these were small and retrospectively designed.26,29,33,34 The Prospective Intraoperative and Perioperative Ophthalmic Imaging with Optical Coherence Tomography (PIONEER) study was the first large, prospective study to evaluate the surgical utility, outcomes, and safety of the microscope-mounted Enfocus iOCT.35 This 2-year study enrolled 531 eyes (256 posterior segment) with iOCT imaging obtained in 98% for multiple indications, the most common being membrane peeling. Eight percent of surgeons changed their surgical management based on their use of iOCT with a 4.9-minute scan session and no adverse events.
The Determination of Feasibility of Intraoperative Spectral-Domain Microscope Combined/Integrated OCT Visualization During en Face Retinal and Ophthalmic Surgery (DISCOVER) study followed the PIONEER study. This was a single-site, multi-surgeon prospective trial using the Enfocus iOCT, the RESCAN-700 microscope-integrated iOCT, and the Cole Eye iOCT systems.28,30,31,36 The 3-year results showed that for 837 eyes (593 posterior segment), Mi-iOCT was feasible with 98% image acquisition. Vitreoretinal surgeons said that Mi-iOCT was useful in 29.2% of cases.36
SURGICAL APPLICATIONS FOR MICROSCOPE-INTEGRATED iOCT
Better understanding of vitreoretinal interface disease and intraoperative changes incurred with surgical techniques and tissue manipulation can influence real-time surgical decision making and possibly lead to improved outcomes. Significant advances in software and hardware of Mi-iOCT systems led to examination of their use for various conditions.
Vitreomacular Traction
In vitreomacular traction (VMT) repair procedures, Mi-iOCT provides real-time assessment of the strength of vitreomacular adhesions and allows visualization of unroofed cysts, subclinical full-thickness macular hole development, and incomplete peeling of membranes. Intraoperative identification of these subclinical changes may alter the immediate surgical approach, such as by prompting the use of gas tamponade, and potentially prevent the need for reoperation.37
Epiretinal Membrane
Mi-iOCT enhances visualization and shows temporary intraoperative changes in the distance between the RPE and the ellipsoid zone and focal areas of retinal elevation at ERM peel initiation sites (Figure 3).38,39 Falkner-Radler et al reported peeling membranes without the use of surgical dyes and only using Mi-iOCT, and they were able to do so in 31% of cases.33
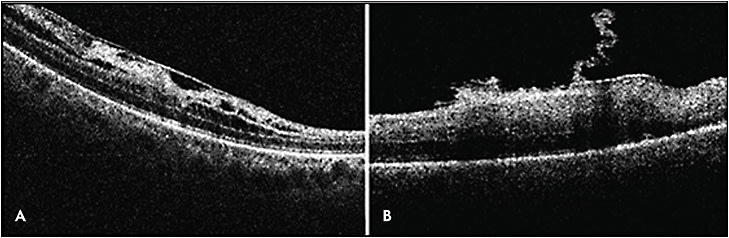
Mi-iOCT has been shown to impact surgeons’ intraoperative decision making for ERM cases. In a subanalysis from the PIONEER trial, iOCT identified occult membranes in 12% of cases and confirmed complete peel contrary to surgeon impression in 9% of cases.40 Other studies have shown a difference in surgeon perception of membranes vs iOCT, where in 12% to 19% of cases the iOCT identified subclinical membranes that required more peeling.35,36,40 In a DISCOVER report, iOCT showed no residual membrane in 40% of cases where the surgeon believed there was more to peel.36 However, whether these intraoperative changes lead to significant functional outcomes remains to be determined.
Macular Hole
In MH surgery, certain configurations of MH and changes in MH geometry seen with Mi-iOCT were associated with anatomical success.22,41 Via the PIONEER trial, Ehlers et al studied intraoperative ILM peeling dynamics and outer retinal and RPE architectural changes. Using iOCT they found that when peeling ILM, increased EZ-RPE height and subretinal hyporeflectivity were negatively correlated with the amount of postoperative subfoveal fluid, which delays time to visual recovery.41,42 Thus, for MH repair, iOCT can be useful not only for performing the ILM peel itself, but also for its prognostic capabilities and assistance in determining if air or long-acting gas tamponade is needed at the end of surgery.
Recently, new surgical techniques have been proposed for chronic, large MH.43,44 Rizzo introduced using amniotic membrane to close MHs and employed iOCT to ensure proper positions of the amniotic membrane plug.43 Mahmoud introduced the use of peripheral autologous retina to plug a MH,44 and iOCT can confirm correct orientation and positioning of the graft through key steps of the case.
Retinal Detachment
In RD surgery, Mi-iOCT aids in detection of residual subretinal fluid (Figure 4A), small retinal breaks, and proliferative vitreoretinopathy membranes, and it can assist in completion of fluid–air exchange. In TRD surgeries, real-time visualization of the planes may also help achieve more precise delamination and segmentation (Figure 4C).
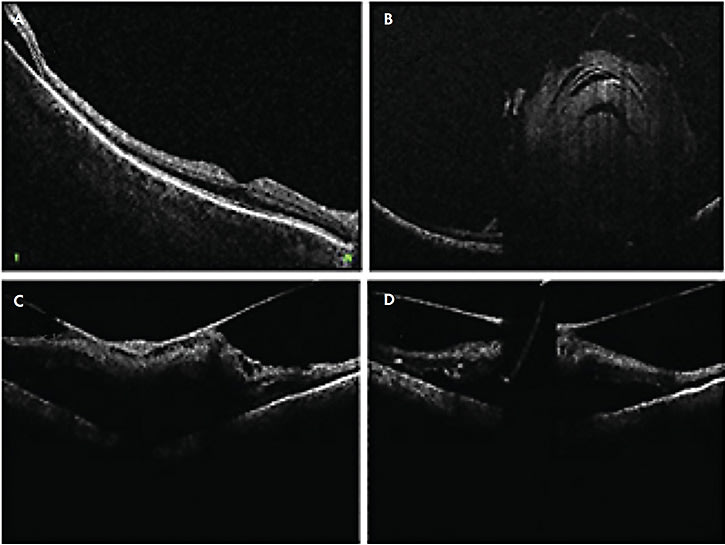
Lee et al used iOCT to show that residual subretinal fluid is possible even after use of perfluorocarbon liquid in eyes that may appear attached under silicone oil.18 In the DISCOVER trial, surgeons changed their plan intraoperatively when using iOCT in 21% of RD cases with examples including optimal retinotomy sites and distinguishing subretinal hemorrhage from fluid.31,36
Subretinal and Submacular Surgery
Subretinal and submacular surgeries are some of the most delicate surgeries not only in ophthalmology, but also in the body, and they require steady and precise movements in the correct microscopic anatomic planes. Subretinal injections can cause devastating complications, such as RD, iatrogenic MH, choroidal hemorrhage, and inadvertent suprachoroidal injection. Intraoperative OCT can be employed to ensure precise anatomic subretinal delivery. It was described for subretinal injection of tissue plasminogen activator injection for submacular hemorrhage in the PIONEER trial.45 Additionally, iOCT can assist in the careful surgical removal of hemorrhages that span multiple layers from the subhyaloid to sub-internal limiting membrane (ILM) and subretinal space.
Intraoperative OCT has been used to ensure proper placement of retinal prosthetic implantation.46 Lastly, as gene therapy has become a reality, successful surgery with precise medication delivery into the subretinal space is vital. Intraoperative OCT has been recommended to help ensure successful delivery of various subretinal gene therapies.47-50
Other Uses
Intraoperative OCT has proven helpful in cases of fundus-obscuring vitreous hemorrhage (VH). Intraoperatively, after clearing the hemorrhage, iOCT helps elucidate epiretinal membranes, macular edema, hyaloid traction, and retinal detachment.51 Intraoperative OCT can help ensure carefully manipulating and removing a dropped IOL lying on the retina.52 The video available with this article at retinalphysician.com shows a case of using iOCT to safely remove a dropped IOL off the surface of the macula. Similarly, iOCT can aid in visualizing the resting place during vitrectomy for intra-ocular foreign bodies or subluxed native lens (Figure 4B). Lastly, iOCT was found useful for surgeries with a uveitis-related diagnosis; iOCT provided beneficial feedback in 85% of eyes for fluocinolone acetonide placement and 81% of chorioretinal biopsies, while altering surgical decision making in 38% of chorioretinal biopsies.53
Intraoperative OCT for Vitreoretinal Surgery from PentaVision on Vimeo.
CHALLENGES AND FUTURE DIRECTIONS
While discoveries and utilities for iOCT are occurring, there are still several limitations to its widespread adaptation. Intraoperative OCT images are currently displayed either on an external monitor, showing larger images that require the surgeon to look away from the microscope, or directly into the surgeon’s field of view via a smaller semitransparent image within the oculars. Advances in Mi-OCT technology are allowing for integration with 3D heads-up viewing systems, allowing the surgeon to view OCT images concurrently with their surgical field on 4K high-definition displays.54 Similarly, iOCT could be used in the novel Clarity surgical headset display system developed by Beyeonics Surgical.55 Though still in development, this headset would project multiple virtual screens onto the surgeon’s retina, with the use of head motions to switch between displays and control functions.
Current iOCT technologies struggle to image peripheral retina and/or show deterioration of the image quality when attempting to do so. This limits the applicability of iOCT for peripheral pathology. There are reports of fiberoptic OCT probes that can be used inside the eye to scan any target tissue including the ora serrata and pars plicata.56-59 By better visualizing the peripheral anatomy and surgical maneuvers, these intraocular probes can potentially uncover additional clinical applications for iOCT.
Another area that needs refinement is the light scattering and shadowing that surgical instruments cause on iOCT. This limits visualization of the instrument-tissue manipulations as well as the underlying tissues (Figures 4C and 4D). The amount of shadowing varies depending on instrument material, configuration, thickness, and relative orientation to the optical axis of the OCT.60 Development of instruments that minimize scatter and shadowing will allow for more precise tissue manipulation. Ehlers et al reported use of semitransparent rigid plastic material instruments that allowed decreased light scatter and improved visibility of adjacent tissue as well as the tissue immediately underlying the instruments.61 Furthermore, development of new software algorithms may assist in software-based processing of the image to minimize shadowing, as well as localize and track the beam to the area of interest to minimize manual readjustments.
One of the most recent advances in iOCT technology is a prototype developed at Duke University that uses swept-source, 4-dimensional (4D) Mi-iOCT visual feedback through real-time volumetric imaging up to 10 volumes per second.62-64 Four-dimensional Mi-iOCT acquires, processes, and renders volumes in real time, which allows for enhanced visualization of tissue deformation and instrument motion and decreases the need for constant tracking of the moving object. Four-dimensional Mi-iOCT is not currently commercially available.
CONCLUSION
Integration of OCT imaging into the vitreoretinal surgeon’s surgical toolbox has made quite an impact over the past decade. Intraoperative OCT has shown to make a positive impact on surgical techniques, intraoperative decision making, and distinguishing anatomic planes in various types of cases including pediatrics, retinal detachments, and macular surgeries. Intraoperative OCT can reduce unnecessary surgical steps, decrease surgical time, and lead to safer and better outcomes. More research is warranted to continue to learn about and improve iOCT applications as well as to analyze long-term impacts of using this technology. As advances and improvements in iOCT technology continue to progress, there way be more widespread acceptance and usage of iOCT technology within the vitreoretinal community. RP
REFERENCES
- Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181.
- Gabriele ML, Wollstein G, Ishikawa H, et al. Three dimensional optical coherence tomography imaging: advantages and advances. Prog Retin Eye Res. 2010;29(6):556-579.
- Jumper MJ, Gallemore RP, McCuen BW, Toth CA. Features of macular hole closure in the early postoperative period using optical coherence tomography. Retina. 2000;20(3):232-237.
- Ripandelli G, Coppe A, Bonini S, et al. Morphological evaluation of full-thickness idiopathic macular holes by optical coherence tomography. Eur J Ophthalmol. 1999;9(3):212-216.
- Mikajiri K, Okada AA, Ohji M, et al. Analysis of vitrectomy for idiopathic macular hole by optical coherence tomography. Am J Ophthalmol. 1999;128(5):655-657.
- Falkner-Radler CI, Glittenberg C, Hagen S, Benesch T, Binder S. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117(4):798-805.
- Massin P, Allouch C, Haouchine B, et al. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery. Am J Ophthalmol. 2000;130(6):732-739.
- Gallemore RP, Jumper MJ, McCuen B, et al. Diagnosis of vitreoretinal adhesions in macular disease with optical coherence tomography. Retina. 2000;20(2):115-120.
- Golas L, Skondra D, Hariprasad SM, Patel RN. Update on intra-operative OCT. Ophthalmol Manag. 2017;21(8):50-53.
- Golas L, Schechet SS, Skondra D, Hariprasad SM. Developments in intraoperative OCT and heads-up assisted surgical viewing. Retin Phys. 2018;15(1):45-48.
- Ung C, Miller JB. Intraoperative optical coherence tomography in vitreoretinal surgery. Sem Ophthalmol. 2019;34(4):312-316.
- Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457-1468.
- Scott AW, Farsiu S, Enyedi LB, Wallace DK, Toth CA. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device. Am J Ophthalmol. 2009;147(2):364-373.
- Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448-2456.
- Chong GT, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127(1):37-44.
- Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010;128(1):57-62.
- Muni RH, Kohly RP, Sohn EH, Lee TC. Hand-held spectral domain optical coherence tomography finding in shaken-baby syndrome. Retina. 2010;30(4 suppl):S45-S50.
- Lee LB, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42 Online:e71-e74.
- Branchini LA, Gurley K, Duker JS, Reichel E. Use of handheld intraoperative spectral-domain optical coherence tomography in a variety of vitreoretinal diseases. Ophthalmic Surg Lasers Imaging. 2016;47(1):49-55.
- Wykoff CC, Berrocal AM, Schefler AC, Uhlhorn SR, Ruggeri M, Hess D. Intraoperative OCT of a full-thickness macular hole before and after internal limiting membrane peeling. Ophthalmic Surg Lasers Imaging. 2010;41(1):7-11.
- Riazi-Esfahani M, Khademi M, Mazloumi M, Khodabandeh A, Riazi-Esfahani H. Macular surgery using intraoperative spectral domain optical coherence tomography. J Ophthalmic Vis Res. 2015;10(3):309-315.
- Ray R, Barañano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118(11):2212-2217.
- Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332-1336.
- Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33(1):232-236.
- Hahn P, Carrasco-Zevallos O, Cunefare D, et al. Intrasurgical human retinal imaging with manual instrument tracking using a microscope-integrated spectral-domain optical coherence tomography device. Transl Vis Sci Technol. 2015;4(4):1.
- Hahn P, Migacz J, O’Connell R, Izatt JA, Toth CA. Unprocessed real-time imaging of vitreoretinal surgical maneuvers using a microscope-integrated spectral-domain optical coherence tomography system. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):213-220.
- Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett. 2010;35(20):3315-3317.
- Runkle A, Srivastava SK, Ehlers JP. Microscope-integrated OCT feasibility and utility with the EnFocus system in the DISCOVER study. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):216-222.
- Pfau M, Michels S, Binder S, Becker MD. Clinical experience with the first commercially available intraoperative optical coherence tomography system. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1001-1008.
- Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: Preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98(10):1329-1332.
- Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER study RESCAN results. JAMA Ophthalmol. 2015;133(10):1124-1132.
- Steven P, Le Blanc C, Velten K, et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013;131(9):1135-1142.
- Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina. 2015;35(10):2100-2106.
- Leisser C, Hackl C, Hirnschall N, et al. Visualizing macular structures during membrane peeling surgery with an intraoperative spectral-domain optical coherence tomography device. Ophthalmic Surg Lasers Imaging Retina. 2016;47(4):328-332.
- Ehlers JP, Dupps WJ, Kaiser PK, et al. The prospective intraoperative and perioperative ophthalmic imaging with optical coherence tomography (PIONEER) study: 2-year results. Am J Ophthalmol. 2014;158(5):999-1007.
- Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated Intraoperative OCT during ophthalmic surgery. Ophthalmology. 2018;125(7):1014-1027.
- Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34(7):1341-1346.
- Ehlers JP, Roth B, Kaiser PK, et al. Assessment of microarchitectural retinal dynamics of epiretinal membrane surgery utilizing intraoperative optical coherence tomography. Presented at: the Macula Society Annual Meeting; 2013; Dana Point, CA.
- Leisser C, Hackl C, Hirnschall N, et al. Visualizing macular structures during membrane peeling surgery with an intraoperative spectral-domain optical coherence tomography device. Ophthalmic Surg Lasers Imaging Retina. 2016;47(4):328-332.
- Ehlers JP, Khan M, Petkovsek D, et al. Outcomes of Intraoperative OCT-assisted epiretinal membrane surgery from the PIONEER Study. Ophthalmol Retina. 2018;2(4):263-267.
- Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34(2):213-221.
- Ehlers JP, Itoh Y, Xu LT, Kaiser PK, Singh RP, Srivastava SK. Factors associated with persistent subfoveal fluid and complete macular hole closure in the PIONEER study. Invest Ophthalmol Vis Sci. 2014;56(2):1141-1146.
- Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:S95-S103.
- Grewal DS, Charles S, Parolini B, Kadonosono K, Mahmoud TH. Autologous retinal transplant for refractory macular holes: multicenter international collaborative study group. Ophthalmology. 2019;126(10):1399-1408.
- Ehlers JP, Petkovsek DS, Yuan A, Singh RP, Srivastava SK. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46(3):327-332.
- Rachitskaya AV, Yuan A, Marino MJ, Reese J, Ehlers JP. Intraoperative OCT imaging of the Argus II retinal prosthesis system. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):999-1003.
- Lam BL, Davis JL, Gregori NZ, et al. Choroideremia gene therapy phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2019;197:65-73.
- Gregori NZ, Lam BL, Davis JL. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina. 2019;39:S9-S12.
- Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308-1316.
- Westenskow PD, Kurihara T, Bravo S, et al. Performing subretinal injections in rodents to deliver retinal pigment epithelium cells in suspension. J Vis Exp. 2015;95:52247.
- Ehlers JP, Griffith JF, Srivastava SK. Intraoperative optical coherence tomography during vitreoretinal surgery for dense vitreous hemorrhage in the PIONEER study. Retina. 2015;35(12):2537-2542.
- Schechet SA, Golas L, Hariprasad SM. Intraoperative optical coherence tomography of a dislocated intraocular lens. J Ophthalmic Vis Res. 2019;14(1):116-117.
- Kumar JB, Ehlers JP, Sharma S, Srivastava SK. Intraoperative OCT for uveitis-related vitreoretinal surgery in the DISCOVER study. Ophthalmol Retina. 2018;2(10):1041-1049.
- Ehlers JP, Uchida A, Srivastava SK. The integrative surgical theater: combining intraoperative optical coherence tomography and 3D digital visualization for vitreoretinal surgery in the DISCOVER study. Retina. 2018;38:S88-S96.
- Loewestein A, Schneider R, Barak A. (2019, February 5). First look: a head-mounted OR display. Rev Ophthalmol. 2019. Available at: https://www.reviewofophthalmology.com/article/first-look-a-headmounted-or-display . Accessed November 20, 2019.
- Han S, Sarunic MV, Wu J, Humayun M, Yang C. Handheld forward-imaging needle endoscope for ophthalmic optical coherence tomography inspection. J Biomed Opt. 2008;13(2):020505.
- Ren J, Gille HK, Wu J, Yang C. Ex vivo optical coherence tomography imaging of collector channels with a scanning endoscopic probe. Invest Ophthalmol Vis Sci. 201;52(7):3921-3925.
- Zhao M, Huang Y, Kang JU. Sapphire ball lens-based fiber probe for common-path optical coherence tomography and its applications in corneal and retinal imaging. Opt Lett. 2012;37(23):4835-4837.
- Asami T, Terasaki H, Ito Y, et al. Development of a fiber-optic optical coherence tomography probe for intraocular use. Invest Ophthalmol Vis Sci. 20165;57(9):OCT568- OCT574.
- Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33(7):1428-1434.
- Ehlers JP, Uchida A, Srivastava SK. Intraoperative optical coherence tomography-compatible surgical instruments for real-time image-guided ophthalmic surgery. Br J Ophthalmol. 2017;101(10):1306-1308.
- Carrasco-Zevallos OM, Keller B, Viehland C, et al. Optical coherence tomography for retinal surgery: perioperative analysis to real-time four-dimensional image-guided surgery. Invest Ophthalmol Vis Sci. 2016;57(9):OCT37-OCT50.
- Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep. 2016;6:31689.
- Gabr H, Chen X, Zevallos-Carrasco OM, et al. Visualization from intraoperative swept-source microscope-integrated optical coherence tomography in vitrectomy for complications of proliferative diabetic retinopathy. Retina. 2018;38:S110-S120.








