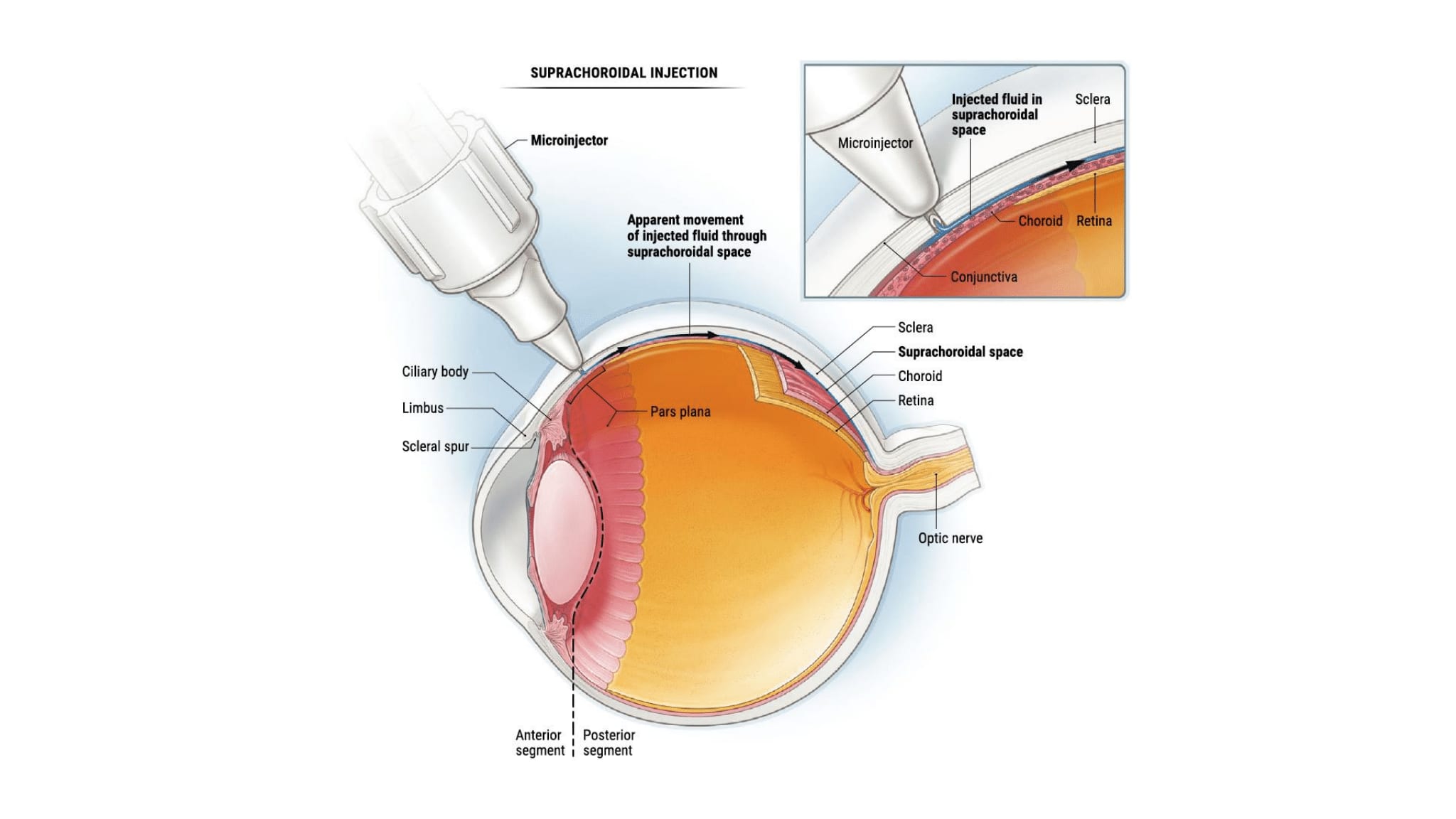
Injection into the suprachoroidal space (SCS) is being developed as a new, more targeted approach to drug delivery with the potential to achieve chorioretinal concentrations 10 times greater than that seen with traditional intravitreal injections (Figure 1).1,2 This drug distribution profile makes SCS injection a contender in the treatment of both retinal and choroidal pathology, with potential future applications in gene therapy3 and ocular oncology.4 In addition to maximizing delivery to the posterior segment, this route of administration also reduces the drug’s exposure to the vitreous and anterior segment.2 This makes SCS injection for steroid delivery particularly tempting. By selectively targeting the posterior segment, suprachoroidal injection has the potential to mitigate steroid’s impact on the anterior segment and thus reduce the rate of intraocular pressure (IOP) increase, a common side effect typically associated with this drug class.
Uveitic Macular Edema
With that in mind, the SCS injection of triamcinolone has been pursued for multiple indications. The phase 3 PEACHTREE trial of suprachoroidal triamcinolone in subjects with uveitic macular edema successfully met its endpoint. In this 6-month trial, 160 patients were randomized 3:2 to receive suprachoroidal trimacinolone vs sham injections at Day 0 and Week 12. The primary endpoint was the proportion of patients gaining ≥15 letters of best-corrected visual acuity (BCVA) at week 24.5
Forty-five of the 96 patients (46.9%) in the treatment group and 10 of the 64 patients (15.6%) in the control group achieved a ≥15 letter gain (P<.001). In addition, the treatment arm also met several secondary anatomic endpoints, including a reduction in macular edema as measured on optical coherence tomography (OCT). Subjects receiving suprachoroidal triamcinolone had a 153 µm mean reduction in central subfield retinal thickness (CST), whereas subjects in the control group had an 18 µm reduction in CST (P<.001).5
Suprachoroidal triamcinolone was well tolerated, with only 7.3% of patients experiencing an IOP rise that required topical therapy. While one should always approach cross-trial comparisons with caution, a recent trial of periocular vs intravitreal steroids for uveitic macular edema (POINT) reported a cumulative 6-month risk of IOP elevation between 26% and 41% for intravitreal triamcinolone and the intravitreal dexamethasone implant, with approximately 30% of patients requiring topical therapy.6 Although head-to-head trials would be needed for a definitive comparison, it would seem that SCS injection of triamcinolone has the potential for lower rates of IOP elevation.
The US Food and Drug Administration (FDA) accepted the new drug application for the suprachoroidal injection of triamcionlone for uveitic macular edema in February, with a Prescription Drug User Fee Act date of October 19, 2019.
Diabetic Macular Edema
It was recently announced that suprachoroidal triamcinolone also demonstrated positive results in the phase 2 TYBEE trial for diabetic macular edema (DME).7,8 In this 6-month trial, a total of 71 treatment-naïve patients with DME were randomized 1:1 either to a combination of suprachoroidal triamcinolone and intravitreal aflibercept at day 0 and week 12 or to monthly intravitreal aflibercept injections until week 12. Patients in the aflibercept arm received further as-needed (PRN) aflibercept treatments at the week 16 and week 20 visits. Patients in the combination arm were eligible for PRN aflibercept at any visit other than those at which they received combination therapy. The criteria for PRN treatment were
- Central subfield retinal thickness ≥340 µm, or
- A 50 µm increase in CST from last visit with either
a. >5 letter decline in BCVA from last visit or
b. >9 letter decline in BCVA from best measurement.7
The primary endpoint, defined as mean change in BCVA from baseline, was met, with both the combination arm (+12.3 letters) and the aflibercept monotherapy arm (+13.5 letters) gaining significant vision by week 24 (P<.001).7 Although vision gains were significant and similar in both groups, the combination arm showed the potential for better anatomic outcomes. Subjects receiving the suprachoroidal triamcinolone in addition to aflibercept demonstrated a greater reduction in CST than aflibercept alone at all time points, with a final reduction of 227 µm compared to 176 µm with monotherapy at week 24 (P=.035).7
These vision and anatomic gains were achieved with fewer treatment visits in the combination arm (2.8) than the aflibercept arm (4.7).8 Significantly fewer PRN treatments were needed with the suprachoroidal trimacinolone-intravitreal aflibercept combination: 78.8% of subjects needed no retreatment, 12.1% need 1 PRN aflibercept, and 9.1% needed 2. This was statistically significant when compared to aflibercept monotherapy, where 58.8%, 14.7%, and 26.5% required 0, 1, and 2 retreatments, respectively (P=.0025).8
The treatment was well tolerated, with no serious adverse events (AEs) attributable to the treatment procedure or medication. Non-IOP related ocular AEs were well balanced. Of note, cataract progression, difficult to definitively assess by the 6-month time point, was seen in 5.6% of the combination group and 2.9% of the aflibercept group. Increased IOP was noted at a higher rate (8.3%) in the combination group compared to the aflibercept group (2.9%).8 The increased IOP was well managed with topical therapy and did not require surgical intervention.
In short, in the phase 2 TYBEE trial, the addition of suprachoroidal triamcinolone to intravitreal aflibercept therapy demonstrated the potential for significant visual gain in the treatment of DME, similar to aflibercept monotherapy. This effect was achieved with improved anatomy and decreased treatment burden compared to aflibercept alone. Additionally, the combination was well tolerated, with a relatively low rate of IOP increase.
Macular Edema in Retinal Vein Occlusion
While phase 2 TANZANITE trial results for the use of SCS injection of triamcinolone for macular edema in retinal vein occlusion (RVO) held promise,9 the top-line results of the subsequent phase 3 SAPPHIRE trial results were recently announced, showing that the primary endpoint was not met. Approximately 50% of subjects in both the combination suprachoroidal triamcinolone and intravitreal aflibercept arm as well as the intravitreal aflibercept monotherapy arm gained ≥15 letters of BCVA at 8 weeks.10 Further development of the combination suprachoroidal triamcinolone treatment for RVO has subsequently been discontinued.
Future Possibilities
Multiple therapeutic compounds could lend themselves well to suprachoroidal delivery. Gene therapy, wherein viral vectors transduce retinal pigment epithelium (RPE) and retinal cells to produce a transgene product, is a therapeutic approach that holds great potential. One crucial aspect to the potential success of gene therapy hinges on the delivery of the vector to the correct anatomic space. The best transduction of the retina to date involves surgical subretinal injection, with its inherent risks. Suprachoroidal injection could potentially circumvent many of those risks, as long as sufficient expression can be obtained from this route.
The feasibility of SCS injection of AAV8 vectors was recently demonstrated in rat and nonhuman primate models.3 While an injection of AAV2 only resulted in a small focal area of transfection, a single injection of AAV8 resulted in expression of the transgene product in the retinal pigment epithelium and photoreceptors throughout a significant portion of the eye (18.9%). A second injection of AAV8 increased this expression (30.5%), indicating the possibility of optimizing future therapy with the number of treatments.3
RGX-314, a vector with an anti-VEGF transgene product currently in a phase 1/2a trial for neovascular age-related macular degeneration (NCT03066258), was then also used in the rodent model. Two or 7 weeks after treatment with suprachoroidal RGX-314, an injection of VEGF165 was given to induce vasodilation and vascular leakage. Twenty-four hours after VEGF165 injection, eyes treated with suprachoroidal RGX-314 had significantly less vasodilation on fundus photography than control eyes (treated with suprachoroidal vehicle), as well as less vascular leakage as measured by vitreous albumin levels. These results were similar to the same dose of the vector delivered subretinally.3
Preclinical models suggest that the SCS injection of gene therapy appears feasible, achieving transduction of the retina and RPE with an effect that could potentially be optimized by the number of injections. More specifically, in a rodent model of human VEGF-mediated disease, the suprachoroidal injection of RGX-314 has shown the possibility of suppressing the pathology typically seen with an upregulation of VEGF. Considering that SCS injection is an office-based procedure that, if successful, would negate the need for a surgical approach, further study seems warranted.
Yet another therapeutic space that could potentially benefit from the use of SCS injection is ocular oncology, specifically in the treatment of choroidal melanoma. With the proven efficacy of radiation therapy to choroidal melanomas often coming at the cost of vision,11 interest in alternative, vision-sparing treatment is high. A novel drug-based approach, in turn, may benefit from the localized, posterior concentration achieved with suprachoroidal injection.
One such agent, AU-011, is a viral-like particle bioconjugate (VPB), a first-in-class compound composed of viral nanoparticles derived from the human papillomavirus (HPV), conjugated with infrared-activated particles.12 The HPV-derived capsids preferentially bind to tumor cells13 where they are then activated with a near-infrared laser, destroying the cell membranes and leading to necrosis. The phase 1b/2 open-label, ascending dose trial of intravitreally injected AU-011 showed that it was well tolerated, with subjects receiving 2 treatments of the maximum dose achieving 93% tumor control rate (13 of 14 subjects).14 A licensing agreement with the goal of delivering AU-011 via SCS injection was recently announced.4
Mechanics of Injection
With FDA approval of suprachoroidal triamcinolone for the treatment of uveitic macular edema and its pending introduction to clinics nationwide, some discussion of the SCS injection technique is warranted. The administration of suprachoroidal triamcinolone does differ somewhat from traditional intravitreal injection. While the preparation of the eye is largely the same, preparing the patient for the first suprachoroidal injection does involve a conversation to communicate that there may be some pressure from the hub of the suprachoroidal injection on the ocular surface, followed by a degree of injection site discomfort in some. This discomfort is typically minor and resolves relatively quickly, without treatment.
As for the injection itself, the injection is performed 4 mm to 5 mm from the limbus with (1) the injector held perpendicular to the scleral plane; (2) while maintaining a constant, firm pressure on the plunger; (3) dimpling down on the sclera with the hub of the needle; and (4) injecting consistently and slowly.
The perpendicularity of the approach is critical, because the length of the suprachoroidal needle is designed to match the thickness of the sclera. Approaching the injection at an angle will likely result in the tip of the needle not having cleared the sclera; with the tip of the needle still within the sclera, the density of the sclera results in a prohibitively high resistance to outflow and prevents injection. By approaching the sclera at a perpendicular angle and maintaining constant, firm pressure, one immediately senses the loss of resistance as the tip of the needle clears the sclera and the medication is administered in the correct anatomic plane. Injecting consistently and slowly throughout this time helps to minimize patient discomfort, while dimpling down on the sclera for approximately 5 seconds after injecting helps prevent reflux of the medication.
During clinical trials, SCS injection was well tolerated by patients. The chance of inadvertent intravitreal injection with this technique, though reported, is rare. If done at the pars plana location as described above, the resulting intravitreal injection of triamcinolone should present no new or unfamiliar challenges. It is even more comforting to note that there was not a single incidence of suprachoroidal hemorrhage at any point of clinical development of this delivery system, regardless of phase or indication.
Conclusion
Suprachoroidal injection better targets the posterior segment and avoids the anterior segment, thus potentially enhancing treatment effect and minimizing side effects. Suprachoroidal injection of triamcinolone has met with positive results in uveitic macular edema and has shown promise in DME. Additionally, interest in suprachoroidal delivery of other potential agents, including gene therapy and light-activated VPB, is high. With the pending clinical introduction of suprachoroidal triamcinolone for uveitis and the possibility of future applications of suprachoroidal injection, familiarity with the injection technique will help prepare retina specialists to continue to evolve and incorporate this new facet to their treatment algorithms.
References
- Patel S, Lin A, Edelhauser H, Prausnitz M. Suprachoroidal drug delivery to the back of the eye using hollow needles. Pharm Res. 2011;28(1):166-176.
- Patel S, Berezovsky D, McCarey B, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433-4441.
- Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019 Aug 13;130.
- Clearside Biomedical. Clearside Biomedical announces license agreement with aura biosciences for suprachoroidal space microinjector designed to optimize ocular oncology drug delivery. https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-license-agreement-aura . July 9, 2019. Accessed August 12, 2019.
- Yeh S. Suprachoroidally Injected CLS-TA improves visual acuity and macular edema in noninfectious uveitis: results of the phase 3 PEACHTREE study. Presented at: American Society of Reinal Specialists; July 25, 2018; Vancouver, Canada.
- Thorne JE, Sugar EA, Holbrook JT, et al; Multicenter Uveitis Steroid Treatment Trial Research Group. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the treatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) trial. Ophthalmology. 2019;126(2):283-295.
- Barakat MR. Suprachoroidal drug delivery for diabetic macular edema. Paper presented at: Retina World Congress; 2019; Fort Lauderdale, FL.
- Khanani A. Suprachoroidal CLS-TA Plus Aflibercept Compared to Aflibercept Monotherapy for Diabetic Macular Edema: Results of the Randomized Phase 2 TYBEE Trial. Paper presented at: Retina World Congress; 2019; Fort Lauderdale, FL.
- Campochiaro PA, Wykoff CC, Brown DM, et al; Tanzanite Study Group. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the Tanzanite study. Ophthalmol Retina. 2018;2(4):320-328.
- Clearside Biomedical. Clearside Biomedical announces SAPPHIRE phase 3 study of combination therapy in retinal vein occlusion did not meet its primary endpoint. https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-sapphire-phase-3-study . November 11, 2018. Accessed July 3, 2019.
- Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostication toolvision loss following episcleral brachytherapy for uveal melanomavision loss following episcleral brachytherapy for uveal melanoma. JAMA Ophthalmol. 2016;134(6):615-620.
- Aura Biosciences. Light-activated AU-011. http://www.aurabiosciences.com/clinical-programs . 2019. Accessed August 12, 2019.
- Kines RC, Cerio RJ, Roberts JN, et al. Human papillomavirus capsids preferentially bind and infect tumor cells. Int J Cancer. 2016;138(4):901-911.
- McCannel TA, Bhavsar A, Capone A, et al. Two year results of a phase 1b/2 open-label clinical trial of AU-011 for the treatment of small to medium choroidal melanoma. Paper presented at: the 2019 annual meeting of the Association for Research in Vision and Ophthalmology; Vancouver, Canada.








