Most retina specialists encounter the occasional patient who has what resembles a pattern dystrophy, or atypical macular degeneration, that doesn’t quite fit into one diagnostic category. Although the convenient label of “pattern dystrophy” often seems adequate, that is not the case with pentosan polysulfate sodium (PPS) maculopathy. This newly described condition masquerades as other maculopathies with a notable exception: it is likely preventable. Here we address some of the frequently asked questions that we have encountered since describing this condition in 2018.1
WHAT IS PENTOSAN POLYSULFATE?
The glycosaminoglycan-like macromolecule pentosan polysulfate sodium (Elmiron; Janssen Pharmaceuticals) serves as a mainstay in the treatment of interstitial cystitis (IC). It received FDA approval for this indication in 1996, and it remains the only FDA-approved oral treatment for IC. Interstitial cystitis is a chronic regional pain syndrome characterized by pain or discomfort in the bladder and pelvic region.2 It affects more than 1 million individuals in the United States, with the majority being female. Although the precise mechanism of action is unclear, PPS is thought to coat the bladder epithelium and protect it against potential irritants.3
HOW STRONG IS THE LINK BETWEEN PPS EXPOSURE AND MACULAR DISEASE?
Emerging data have demonstrated that the link between PPS and this characteristic maculopathy is quite strong. In our initial report, we described macular pigmentary changes in 6 PPS-exposed patients seen by a single clinician. All of these patients reported chronic exposure to the drug. Although these findings were suggestive of a drug toxicity, they did not exclude the possibility that IC itself or one of its many off-label therapies was responsible for the macular findings.
To further investigate this, we performed a retrospective cross-sectional study evaluating all patients with IC seen at the Emory Eye Center over a 4-year period. We identified 219 patients with IC, 80 of whom had prior exposure to PPS. Masked reviewers graded available imaging for all IC patients, and identified 14 cases of the characteristic maculopathy. All cases occurred in the PPS-exposed group. Not a single case of this maculopathy was observed in patients who had not taken PPS. Among all drug exposures and other potential risk factors, PPS exposure was the only factor that was significantly associated with this unique maculopathy.4
On a broader scale, we also performed a retrospective matched-cohort study of claims data from a large US insurer. We evaluated the incidence of a new “macular disease” diagnosis at 5 and 7 years after initial exposure to PPS, as compared to unexposed age- and sex-matched controls. In a multivariate model, PPS exposure conferred a trend for increased odds of a new diagnosis of macular disease at 5 years, and a statistically significant increase in odds at 7 years (unpublished data).
These findings strongly implicate PPS, although further work is needed to firmly establish causality. We continue to explore this question with larger studies in humans as well as in the laboratory with animal models.
HOW COMMON IS THIS PROBLEM?
Although we don’t have a robust estimate of the incidence of PPS maculopathy, a primary concern at present is that this medication has been on the market for decades. Hundreds of thousands of patients have been exposed to it, and there are likely many patients with PPS maculopathy who have yet to be identified.
Anecdotally, at our tertiary care institution, approximately half of our retina faculty have seen at least 1 patient with PPS maculopathy over the past 4 years. Most affected patients presented with a referral diagnosis of pattern dystrophy or AMD. A disproportionate number of cases were seen via outside referral to our ophthalmic genetics service.
WHAT ARE THE KEY CLINICAL FEATURES OF PPS MACULOPATHY?
Pentosan polysulfate maculopathy appears to have a fairly well-defined clinical spectrum, as observed in a retrospective study of 70 eyes of 35 patients across 4 institutions.5 All patients reported chronic PPS exposure (median 14.5 years, range 3-21.9 years), although 1 patient developed symptoms several years after a relatively short 3-year course of PPS. Importantly, most of these patients experienced years of visual symptoms prior to the diagnosis, suggesting that the exposure threshold for disease onset may be lower than expected.
Affected patients often presented with blurred vision (49%) and prolonged dark adaptation (49%) in spite of relatively preserved visual acuity (median Snellen VA 20/25, range 20/15-20/400). Only 10 eyes presented with visual acuity worse than 20/40, and 2 eyes presented with visual acuity worse than 20/200. Nonetheless, we have found these patients to express great frustration with their functional deficits.
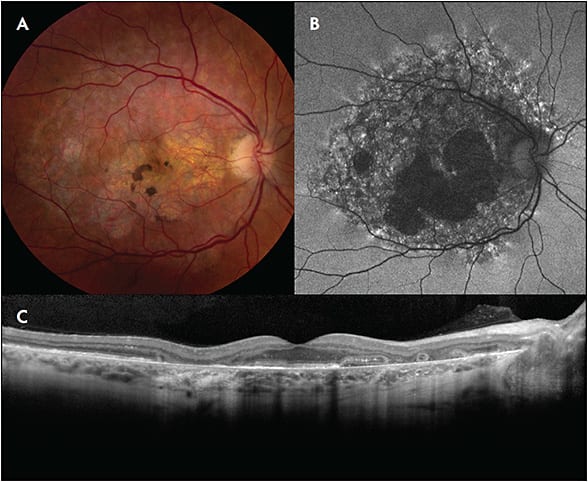
On examination and color fundus photography, patients often exhibited relatively nondescript fundus changes, typically with paracentral pigment clumps amidst a background of yellow-orange subretinal deposits. The fundus findings were most striking on autofluorescence and near-infrared reflectance imaging. Autofluorescence imaging typically demonstrated a densely packed pattern of hyperautofluorescent and hypoautofluorescent spots that was centered on and involved the fovea. Fundus alterations were typically confined to the posterior pole, although they occasionally extended to the retinal periphery, often in a reticular pattern. Optical coherence tomography (OCT) demonstrated nodular lesions at the level of the retinal pigment epithelium that correspond to the hyperpigmented macular spots.
Some eyes developed RPE atrophy, which involved the central fovea in advanced cases. A total of 9 eyes of 6 patients manifested cystoid macular edema, which responded well to a range of topical and intravitreal therapies. One eye had what was thought to be choroidal neovascularization.
Longitudinal evaluation in a limited number of cases demonstrated this to be a fairly dynamic disease process. The disease can extend peripherally with time, and pigmented macular spots appeared to give way to RPE atrophy.
SCREENING AND TREATMENT
Although it is premature to institute formal screening guidelines, we have adopted the following approach in our clinical practice: For patients initiating a long-term course of PPS therapy, we recommend that they discuss dosing strategies with their prescriber, with the goal of using the lowest necessary dose for IC symptom control, and with attempts to reduce exposure duration when possible. We recommend obtaining a baseline examination with comprehensive fundus imaging (color fundus photography, fundus autofluorescence imaging, and OCT). Subsequently, we advise that patients undergo annual examination with repeat imaging starting at 5 years after PPS initiation.
Providers should exercise caution in prescribing PPS to patients with comorbid macular disease such as AMD, which may confer higher susceptibility to a toxic maculopathy. Patients with potentially elevated risk, including those with an atypical dosing regimen, those with a history of smoking or macular disease, as well as those with comorbidities involving hepatic, renal, or splenic function, may benefit from more frequent screening examinations.
For patients diagnosed with PPS maculopathy, we recommend drug cessation and coordination with the prescribing physician to explore alternative regimens for IC management.6 Although we do not fully understand the natural history of this condition, we caution affected patients that visual symptoms may persist, and possibly continue to worsen, even after drug cessation.
CONCLUSIONS
Given the growing evidence for a PPS-induced macular toxicity, retina specialists have a new role to identify affected patients and prevent others from developing this vision-threatening condition. These patients may be easily misdiagnosed. However, when presented with a case of a so-called pattern dystrophy or atypical AMD, a detailed medication history and multimodal imaging should help reveal the diagnosis.7 We look forward to ongoing investigations to improve our understanding of the pathobiology, incidence, and prognosis of this unique maculopathy. RP
REFERENCES
- Pearce W, Chen R, Jain N. Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium. Ophthalmology. 2018;125(11):1793-1802.
- Nickel JC, Moldwin R. FDA BRUDAC 2018 Criteria for Interstitial Cystitis/Bladder Pain Syndrome Clinical Trials: Future Direction for Research. J Urol. 2018;200(1):39-42.
- Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(5A Suppl):93-99.
- Hanif AM, Shah R, Yan J, et al. Strength of association between pentosan polysulfate and a novel maculopathy. Ophthalmology. April 18, 2019. [Epub ahead of print].
- Hanif A, Taylor S, Armenti S, et al. Expanded clinical spectrum of pentosan polysulfate sodium-associated pigmentary maculopathy. Paper presented at: Association for Research in Vision and Ophthalmology; April 30, 2019; Vancouver, BC, Canada.
- Giusto LL, Zahner PM, Shoskes DA. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 2018;19(10):1097-1108.
- Hanif AM, Yan J, Jain N. Pattern dystrophy: an imprecise diagnosis in the age of precision medicine. Int Ophthalmol Clin. 2019;59(1):173-194.








