Diabetic retinopathy (DR) is the leading cause of preventable vision loss in adults aged 20 to 74 years, affecting 1 out of every 12 people with diabetes.1 Diabetic macular edema (DME), a complication of DR, is the primary cause of vision loss in both proliferative DR (PDR) and nonproliferative DR (NPDR).2 Diabetic macular edema is the accumulation of extracellular fluid and lipoproteins in the retina due to damaged and hyperpermeable retinal vessels.3,4 Fourteen percent to 25% of people with type 2 diabetes and 20% with type 1 diabetes will develop DME during a 10-year follow up.5,6 If left untreated, DME will progress from blurred and distorted vision to severe vision loss. In 1985, the Early Treatment Diabetic Retinopathy Study (ETDRS) established macular laser photocoagulation as the standard of care for DME. The data from ETDRS found evidence that laser photocoagulation could slow the progression of vision loss in patients with clinically significant macular edema by up to 50%.7 Despite the clinical effectiveness of slowing the progression of vision loss, laser photocoagulation demonstrated little ability to restore vision and repair retinal damage.8
The discovery of anti-vascular endothelial growth factor (anti-VEGF) therapies opened the door to a new potential treatment for DME. Because VEGF is the primary cytokine involved in the hyperpermeability of retinal vessels,9,10 it was hypothesized that anti-VEGF therapy could be an effective treatment for DME.11 In 2011, ranibizumab (Lucentis; Genentech), an intravitreal anti-VEGF antibody fragment, entered clinical trials for the treatment of vision loss from DME. Investigators hoped to find a treatment that would not only slow the progression of vision loss, but also rapidly improve visual acuity. The efficacy and safety of ranibizumab was explored in the phase 3 RIDE and RISE trials (Figure 1). In addition, studies of aflibercept with the phase 3 VIVID and VISTA trials demonstrated the superiority of anti-VEGF over laser treatment for DME. Finally, off-label use of bevacizumab (Avastin; Genentech) now studied in Protocol T by the DRCR Retina Network further validated anti-VEGF therapy as the primary treatment for DME.12
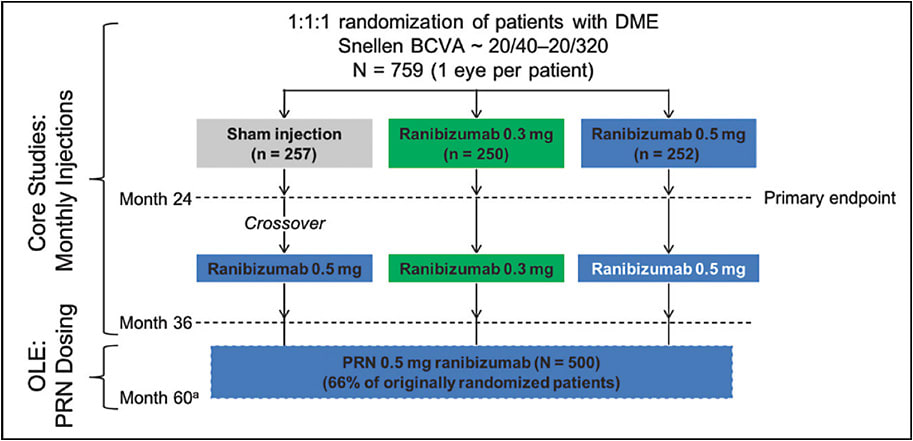
The field of DME treatment has drastically changed. Anti-VEGF therapy is quickly replacing laser photocoagulation as the standard of care for DME. With such rapid innovation and transformation in the landscape of DME therapy, it is important to be wary of some common misconceptions. Following are 5 myths to be aware of when using anti-VEGF to treat patients with DME.
MYTH 1: TREATMENT DEFERRAL WILL NOT AFFECT VISION GAIN OUTCOMES
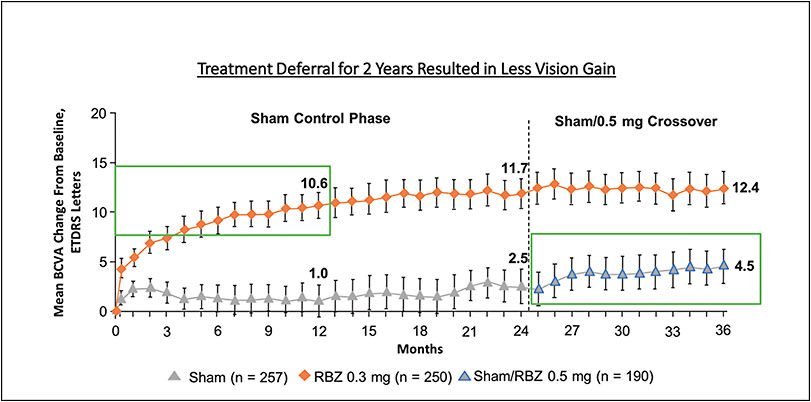
The RIDE/RISE trials revealed significantly less visual acuity (VA) gains in the sham/0.5 mg crossover group (sham injections for 24 months followed by 12 months of 0.5 mg monthly injections of ranibizumab) compared to the 0.3 mg and 0.5 mg ranibizumab group. The sham/0.5mg crossover group resulted in an average 4.5 ETDRS letter gain, while immediate treatment resulted in an average 10.6 (for the 0.3 mg group) and 11.1 (for the 0.5 mg group) letter gain (Figure 2). This might be due to the chronicity of fluid and its detriment on anatomic restoration. For example, the sham/0.5 mg crossover group demonstrated an average reduction of 98.4 μm in central foveal thickness (CFT) on optical coherence tomography (OCT) whereas the 0.3 mg ranibizumab therapy group demonstrated a reduction of 237.9 μm, and the 0.5 mg group demonstrated a reduction of 249.3 μm.13
In the VISTA and VIVID trials, a subgroup of patients in the laser control group began intravitreal injections of aflibercept (Eylea; Regeneron) after 24 weeks if they met the pre-established criteria of worsening DME. In addition to laser treatment, this subgroup of patients received 5 doses of monthly 2 mg intravitreal aflibercept injections (IAI) followed by bimonthly 2 mg IAI until the end of the study. At week 100, the patients in the laser control group who did not demonstrate worsening DME were switched to a PRN dosing regimen of IAI therapy. Grouping together the patients who received injections due to worsening DME after week 24 and the patients who received injections after week 100, a subgroup of patients that began aflibercept treatment after at least a 24-week treatment delay can be identified. The data revealed significantly better visual outcomes in the groups receiving IAI 2 mg every 4 weeks (2q4) and IAI 2 mg every 8 weeks (2q8).
This suggests that a treatment delay of least 24 weeks can still improve vision; however, it will be to a lesser extent than if the treatment were immediately initiated. It is important to note that the between-group difference in the VISTA/VIVID trials (24-week treatment delay) was less than the between-group difference in the RIDE/RISE trials (24-month treatment delay). Therefore, a longer treatment delay will result in less vision gain compared to a shorter treatment delay. Additionally, unlike the 24-month ranibizumab treatment delay, a 24-week aflibercept treatment delay did not result in significantly less CFT reduction. At 148 weeks, the laser control group (including the treatment-delay subgroup) demonstrated an average reduction of 218.86 μm in CFT, whereas the IAI 2q4 group demonstrated reduction in CFT of 222.4 μm and the IAI 2q8 group demonstrated reduction in CFT of 221.3 μm. These data suggest that treatment should be initiated as soon as possible to maximize potential vision gain outcomes and CFT reduction.14
MYTH 2: PRIOR TREATMENT WITH LASER,STEROIDS, OR ANTI-VEGF AFFECTS OUTCOMES
In a subanalysis of the RIDE/RISE trials, 76% of patients received prior laser and/or steroid treatment (laser only, n=346; laser plus steroid, n=218). Visual acuity improvements at 24 months were similar across subgroups (treatment naïve, laser only, laser plus steroid) receiving ranibizumab. In the 0.3 mg ranibizumab group, the treatment-naïve group had an average 12.3-letter gain, the laser-only group had an average 11.5-letter gain, and the laser plus steroid group had an average 11.1-letter gain. In the 0.5 mg ranibizumab group, the treatment-naïve group had an average 11.9-letter gain, the laser-only group had an average 11.7-letter gain, and the laser and steroid group had an average 12.7-letter gain. These data suggest prior treatment did not significantly affect the extent of visual acuity improvement. Additionally, retinal thickness reduction was similar across subgroups, with a mean change from baseline at month 24 ranging from -241.6 to -290.5 μm.15
Within the VISTA and VIVID studies after 52 weeks of aflibercept treatment, there was no significant difference between the subgroup of patients treated with an anti-VEGF agent prior to enrollment and the subgroup of patients who were anti-VEGF naïve prior to enrollment. In VISTA, patients with prior anti-VEGF treatment who received IAI 2q4 demonstrated a 10.7 mean BCVA change while patients who were anti-VEGF naïve demonstrated a 13.9 mean BCVA change. In VIVID, patients with prior anti-VEGF treatment who received IAI 2q4 demonstrated a 14.4 mean BCVA change while patients who were anti-VEGF naïve demonstrated a 10.2 mean BCVA change. This suggests prior anti-VEGF exposure does not significantly affect vision gain outcomes of aflibercept (Figure 3).16

MYTH 3: BASELINE HBA1C AND MACULAR NONPERFUSION AFFECT VISUAL GAINS WITH ANTI-VEGF
Despite being a well-known risk factor for PDR, HbA1c levels were not found to be predictive of progression to PDR when treated with ranibizumab.17 When ranibizumab treatment groups were separated into subgroups based on HbA1c levels, all subgroups demonstrated similar improvement in VA from baseline.18 Unlike HbA1c levels, macular capillary nonperfusion was predictive of progression to PDR; however, it was not predictive of VA improvement. In an analysis of the pooled 0.3mg/0.5mg ranibizumab group data, the subgroup of patients without baseline macular nonperfusion demonstrated an average 12.3-letter gain while the subgroup of patients with macular capillary perfusion demonstrated an average 14.5-letter gain (P=.1043 for difference between groups). Therefore, baseline macular nonperfusion was not found to significantly affect the extent of visual acuity improvement.17
MYTH 4: ANTI-VEGF CANNOT IMPROVE DIABETIC RETINOPATHY
Monthly ranibizumab injections has been found to be an effective treatment for PDR and NPDR.19 In the groups treated with 0.3 mg ranibizumab, 37.2% experienced a 2-step or greater improvement in diabetic retinopathy severity score (DRSS), and 13.2% experienced a 3-step or greater improvement.20
Aflibercept demonstrated similar DR improvement in the VIVID and VISTA trials at 100 weeks. In both VISTA and VIVID, the IAI 2q4 and 2q8 groups had significantly higher proportions of patients who experienced at least a 2-step improvement in the DRSS score than the laser control group. In VISTA, 37.0% of the IAI 2q4 group and 37.1% of IAI 2q8 group had at least a 2-step improvement compared to 15.6% of the laser control group (37.0% and 37.1% vs. 15.6%; P<.0001 for both). In VIVID, 29.3% of the IAI 2q4 group and 32.6% of the IAI 2q8 group had at least a 2-step improvement, whereas 8.2% of the laser control group had at least a 2-step improvement (P=.0004 for IAI 2q4 and P<.0001 for IAI 2q8).21 Anti-VEGF therapy is effective not only in treating DME, but also in reversing the severity of DR.
MYTH 5: MONTHLY THERAPY IS NEEDED TO MAINTAIN OR ACHIEVE VISION GAINS
After 36 months, patients can be switched to a PRN dosing regimen to maintain vision outcomes. At the end of 36 months, the RIDE/RISE trials continued as an open-label extension, and patients were given ranibizumab as needed. An average of 4.5 injections were administered over an average 14.1-month follow-up period. Approximately 25% of patients required no further injections to maintain these outcomes. Despite a significant decrease in treatment frequency, mean VA improvements were maintained. It is important to note that the sham/0.5 mg crossover group that received 12 months of monthly injections demonstrated improvement but not to the same degree as the 0.3 mg and 0.5 mg group that received 36 monthly injections. For the sham/0.5 mg crossover group, treatment after 12 monthly injections solely maintained VA improvement, highlighting the need for early intervention to maximize potential vision gain.
Data from the VIVID and VISTA trials further support the notion that monthly injections are not needed to achieve similar vision gain outcomes. Analysis of mean BCVA gain at 148 weeks revealed no significant difference between the IAI 2q4 and IAI 2q8 groups. In VISTA, mean BCVA gain was 10.4 in the IAI 2q4 group and 10.5 letters in the IAI 2q8 group, and in VIVID, mean BCVA gain was 10.3 in the IAI 2q4 group and 11.7 letters in the IAI 2q8 group. The similarity in vision gains suggest bimonthly injections are as effective as monthly injections.14
CONCLUSION
The RIDE/RISE and VIVID/VISTA trials were pivotal breakthroughs for DME treatment. In addition to demonstrating the effectiveness and sustainability of anti-VEGF therapy for vision loss from DME, these studies addressed several common myths of anti-VEGF treatment. Using a systematic scientific approach, substantial data were gathered and interpreted to understand the true effects and outcomes of anti-VEGF therapy. Regardless of prior treatments, HbA1c levels, and baseline nonperfusion, anti-VEGF can effectively treat DME. In addition to improving visual acuity and reducing retinal thickness, anti-VEGF can improve DR. After mandated monthly or bimonthly intravitreal injections, patients can switch to PRN dosage while maintaining outcomes. Bevacizumab, ranibizumab, and aflibercept are effective, sustainable, multifunctional therapeutic agents that are transforming the way DME is treated. With early intervention, anti-VEGF can lead to better outcomes and improve the quality of life of thousands of patients. RP
REFERENCES
- Kempen JH, O’Colmain BJ, Leske MC, et al; Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552-563.
- Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340.
- Ferris FL, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28:452-461.
- Singh A, Stewart JM. Pathophysiology of diabetic macular edema. Int Ophthalmol Clin. 2009;49(2):1-11.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124-136.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796-1806.
- Nguyen QD, Brown DM, Marcus DM, et al; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801.
- Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408-2413.
- Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820-1828.
- Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961-969.e4.
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203.
- Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022.
- Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2016;123(11):2376-2385.
- Sara J. Haug, Beiying Ding, Na Lu, Ivaylo Stoilov. Ranibizumab for DME following laser and/or steroid treatments: a RIDE/RISE subanalysis. Invest Ophthalmol Vis Sci. 2015;56(7):1759-1759.
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254.
- Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367-374.
- Singh RP, Habbu K, Ehlers JP, Lansang MC, Hill L, Stoilov I. The impact of systemic factors on clinical response to ranibizumab for diabetic macular edema. Ophthalmology. 2016;123(7):1581-1587.
- Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab Induces Regression of Diabetic Retinopathy in Most Patients at High Risk of Progression to Proliferative Diabetic Retinopathy. Ophthalmol Retina. 2018;2(10):997-1009.
- Stewart MW. A review of ranibizumab for the treatment of diabetic retinopathy. Ophthalmol Ther. 2017;6(1):33-47.
- Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044-2052.








