In 2019, our armamentarium for treatment of diabetic retinopathy (DR) ranges from anti-vascular endothelial growth factor (anti-VEGF) intravitreous injections and steroid implants to laser photocoagulation and surgical intervention. For the vast majority of our patients, the choice of treatment pivots primarily around laser and an anti-VEGF drug. For diabetic macular edema (DME), an anti-VEGF drug is usually the primary treatment choice, although focal laser is also a consideration. Anti-VEGF therapy was shown to be better than focal laser for DME in several studies.1-7 For proliferative diabetic retinopathy (PDR), the treatment options are more diverse and include laser photocoagulation, anti-VEGF intravitreous injections,8-10 pars plana vitrectomy, and combination treatments. This article will focus on anti-VEGF and laser interventions for DME and PDR. Factors to consider when selecting a therapy include patient compliance, patient support systems, visual acuity and, of course, individual ocular characteristics. Personalization of the treatment plan is key to achieving visual and anatomic success in the management of DR.
PATIENT-RELATED FACTORS
The success of any treatment paradigm hinges upon patient compliance. Anti-VEGF strategies generally require more intensive treatment plans than laser photocoagulation. Hence, compliance with follow-up is even more critical in these patients. Patient compliance is related to a patient’s general health status, social network, and available transportation to the clinic as well as the patient’s intrinsic motivation to be compliant. Therefore, the review of systems and social history are important components of the patient’s medical history. One should discuss both work-related (ability to take time off work, work-related travel) and social and family support issues (including availability of family or public transport for office visits) with the patient at the initial visit when deciding upon a treatment plan. It is important to convey the anticipated number of treatments and office visits very clearly to the patient and his or her family. For instance, for DME, I discuss the DRCR protocol I anti-VEGF results and treatment paradigm, which showed an expected number of 9 injections in year 1, 5 to 6 injections in year 2, and 2 to 3 injections in year 3 for patients with DME when treated with ranibizumab.2,3 Untoward outcomes in the face of noncompliance should be clearly explained and perhaps illustrated (via fundus photos) to the patient.
A patient who has multiple systemic diseases, which may negatively impact the ability to keep scheduled visits, would not be an ideal candidate for anti-VEGF therapy. Although missed visits can result in devastating outcomes with either anti-VEGF or laser treatment, incomplete anti-VEGF treatment of DR is more likely to result in poorer outcomes than a missed follow-up visit in a patient who received laser treatment for PDR.11-13 Research has documented poor outcomes of missed follow-up visits with anti-VEGF treatment. Certain factors (eg, nonwhite race, lower socioeconomic status, lower baseline visual acuity) were associated with a higher risk for missed visits.11 Patients must be clearly informed of the consequences of noncompliance prior to beginning any treatment regimen. Disclussion should also include the importance of good control of blood glucose, blood pressure, serum cholesterol, and hemoglobin A1c levels. I routinely discuss these factors with my diabetic patients at each of their visits.
Another consideration in deciding whether to choose laser or anti-VEGF therapy is the presence or absence of recent cardiovascular events. Patients who suffered a recent stroke or heart attack (within 2 months) were excluded from the clinical trials using anti-VEGF therapy for DR. I consider these events as contraindications to using an anti-VEGF and do not recommend anti-VEGF therapy to such affected patients. The risk of adverse events was not studied in these patients who are already known to be at a higher risk for a subsequent cerebrovascular event or cardiac event.14
I consider use of an anti-VEGF in such a patient beginning 3 months after a cerebrovascular or cardiac event, provided the patients is medically stable. Communication with the patient’s primary care physician or specialist is key to achieving success and mitigating risk.
MANAGEMENT OF DIABETIC MACULAR EDEMA
When visual acuity is impaired and DME is subfoveal, anti-VEGF therapy is the straightforward treatment of choice, barring any of the aforementioned contraindications. When baseline visual acuity is decreased, Protocol I of the DRCR Retina Network showed us that anti-VEGF therapy results in significant visual acuity gains, whereas laser therapy did not.1,2 The pivotal DME trials RIDE and RISE using ranibizumab (Lucentis; Genentech)3 and VIVID and VISTA using aflibercept (Eylea; Regeneron)4 also achieved similar outcomes. BOLT showed similar results for bevacizumab (Avastin; Genentech).5 Unless the patient refuses this treatment or if there is a contraindication to use of an anti-VEGF injection, anti-VEGF offers the patient the best chance at improvement of visual acuity. Protocol T showed equivalent outcomes with any of the 3 anti-VEGF drugs for patients with good baseline visual acuity. However, when visual acuity was 20/50 or worse, aflibercept resulted in the best visual acuity outcome overall. At the end of year 1, aflibercept was better than ranibizumab or bevacizumab, although by year 2 it was better only against bevacizumab. Analysis of the area under the curve analysis still showed aflibercept had better overall vision during the 2 years.6,7 Also, the OCT data showed that ranibizumab and aflibercept had more efficacy in drying the retina than bevacizumab; patients treated with bevacizumab were more likely to have persistent fluid.
When performing anti-VEGF therapy for DME, it is important to continue therapy even when it may initially appear on OCT that there is no improvement in retinal anatomy or vision. Studies have shown that patients can and do still improve at 12 months even if there appeared to be limited or no response at 3 months after initiation of treatment. In addition, rates of visual acuity improvement were not significantly different despite the presence of persistent DME as long as eyes were receiving anti-VEGF therapy according to the DRCR protocol.15,16 The question of switching anti-VEGF treatment for lack or poor response to another anti-VEGF is currently being investigated by the DRCR Retina Network. One useful strategy, for when there is no apparent response 4 weeks following an anti-VEGF treatment, is to re-evaluate the patient 2 weeks following an anti-VEGF injection and determine whether the DME has improved.
Protocol V showed that one can initially observe patients with visual acuity 20/25 or better without detrimental effect as long as they are followed and treated when visual acuity decreases.17 It is important to remember that this protocol allowed for treatment (with aflibercept) if visual acuity worsened in the observation or laser groups by 10 letters (≥2 lines on an eye chart) at any visit or by 5 to 9 letters (1-2 lines) at 2 consecutive visits. This occurred in 25% (60/240) of eyes in the laser photocoagulation group and 34% (80/236) of eyes in the observation group. Thus, continued follow-up is needed for these eyes when treatment is deferred. This point cannot be overemphasized. Analyses of outcomes by baseline severity of OCT central subfield thickness and proximity to the foveal center are being performed at present.
Although there was no difference in loss of 5 or more letters or in the mean visual acuity at 2 years, in the prespecified, exploratory, longitudinal analysis, mean change in visual acuity letter score over 2 years (area under the curve) was better for aflibercept than for laser and also for aflibercept than for observation: 1.5 letters (SD, 4.0) with aflibercept, 0.0 letters (SD, 3.9) with laser photocoagulation, and −0.4 letters (SD, 4.2) with observation (aflibercept vs laser photocoagulation mean difference in visual acuity letters 1.9 [95% CI, 1.0-2.8; P<.001]; aflibercept vs observation mean difference in visual acuity letters 2.1 [95% CI, 1.1-3.1; P<.001]; laser photocoagulation vs observation mean difference in visual acuity letters 0.2 [95% CI, −0.7-1.0; P=.73]). However, these differences are not likely to be clinically relevant because the mean visual acuity was about 20/20 for all groups.
The key point for these eyes with good visual acuity is to follow the patients carefully if choosing observation and to treat when a drop in visual acuity occurs. It is important to remember that in this protocol, patients in the observation group were followed initially at 8 and 16 weeks and then every 16 weeks unless visual acuity or OCT worsened. One could argue that if patients cannot be expected to reliably return for follow-up, then treatment could be initiated in the hopes of resolving edema before acuity may be adversely affected. Loss to follow-up is not insignificant in patients who receive anti-VEGF for DME.11 It is also important to remember that there is a disconnect between change in visual acuity and change in OCT. Change in OCT cannot serve as a reliable surrogate for vision in DME.18
An added benefit of anti-VEGF therapy for DME is the reduction of the ETDRS level of DR. This has been shown to persist at 3 and 5 years, despite the concurrent reduction in number of anti-VEGF intravitreous injections for DME over time.19 Whether the regression of the NPDR translates to a true change in the biology of the disease remains to be shown. However, it is considered another positive factor in favor of anti-VEGF therapy for DME over laser.
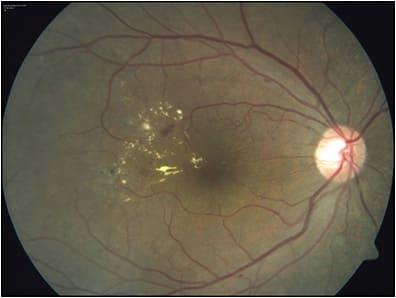
There are cases in which focal laser is a treatment of choice. For DME without visual loss and without foveal edema or foveal-threatening edema, laser is a good option. Indeed, the ETDRS showed good visual and anatomic outcomes were achieved when baseline acuity was good.20 Cases with an extrafoveal circinate ring are ideal for laser photocoagulation (Figure 1). Situations with hard exudates from a circinate ring just involving the fovea when associated with extrafoveal microaneurysms are also good candidates for focal laser. When performed, laser photocoagulation should be delivered in a light-modified ETDRS fashion. The ETDRS showed that visual acuity is not a criterion for determination of treatment when clinically significant DME was present. Thus 20/20 eyes in which thickening threatens the center should be treated with laser. This contrasts with the current recommendations from Protocol V when anti-VEGF is being considered. Another situation in which laser can be considered is for patients in whom an anti-VEGF is contraindicated (recent stroke or myocardial infarction). Steroids are another option, particularly for chronic DME, but that discussion is beyond the scope of this article.
MANAGEMENT OF PROLIFERATIVE DIABETIC RETINOPATHY
For PDR, anti-VEGFs were shown to have similar anatomic outcomes to that of PRP. Both treatments resulted in similar rates of control of neovascularization and visual acuity outcomes. However, in year 1, the visual field was significantly better with anti-VEGF than PRP. Rates of developing DME or vitreous hemorrhage or need for pars plana vitrectomy were also lower with anti-VEGF therapy than with laser.8,9 The anti-VEGF benefit was greater in patients who had higher baseline mean arterial pressure, and in those with more advanced PDR.21 Five-year results showed visual acuity of 20/25 for both treatment groups, although ranibizumab-treated eyes had lower rates of developing DME and less visual field loss.9 Interestingly, the anti-VEGF eyes did show field loss at 5 years, although not to the same extent as the PRP eyes, suggesting possibly that PDR itself results in visual field loss.
In Protocol S, intravitreous ranibizumab (0.5 mg) was given monthly for 6 months unless resolution of PDR was achieved after 4 injections. After 6 months, injections could be deferred if neovascularization was stable over 3 consecutive visits (sustained stability). If neovascularization worsened, monthly treatment resumed. For failure or futility criteria, PRP could be initiated. Additional PRP was required in 45% of patients by the end of 2 years for eyes given PRP initially. Thus, follow-up is necessary for PRP.9 Follow-up in the anti-VEGF group showed that the median number of injections between 6 months and 2 years was 4 for eyes with resolved neovascularization vs 7 for eyes without resolved neovascularization at 6 months (P<.001). For eyes in which a deferral of anti-VEGF therapy was possible (93%), 62% (42 of 68) resumed injections within 16 weeks after deferral. Thus, at least quarterly follow-up is needed in this group. A post-hoc analysis of the data showed good PDR control when the paradigm was followed.22
Furthermore, the CLARITY study, which compared aflibercept to PRP for PDR eyes without macular edema, found 65% of PRP patients required additional PRP when monitored every 8 weeks over 52 weeks.10 In this noninferiority phase 2b study, aflibercept was given at baseline and then every 4 weeks for a total of 3 loading doses. Then, aflibercept treatment was given as needed, during follow-up intervals of 4 weeks. PRP treated eyes were assessed at week 12 and then every 8 weeks. Visual acuity results were superior for aflibercept over PRP; mean change in best corrected visual acuity was -3.0 letters (standard error 0.7) for PRP and 1.1 letters (standard error 0.6) for aflibercept (difference=3.9; 95% CI, 2.3-5.6; P<.0001). In addition, there were fewer eyes that developed DME as well as vitreous hemorrhage (9% versus. 18%) in the aflibercept group. There were more eyes with total regression of PDR (30% difference, P<.0001) when treated with aflibercept than with PRP. Low-luminance visual acuity was better in the aflibercept-treated eyes than the PRP eyes. Patient satisfaction score surveys showed patients preferred aflibercept to PRP. Regression of PDR with anti-VEGF is frequently seen (Figure 2). In Protocol S, 47% of patients at 2 years and 46% at 5 years had a 2-step regression in the severity of DR. CLARITY also showed a significant proportion of eyes with improvement in DR levels when treated with aflibercept. Whether there is truly a change in the biology of the disease remains to be determined.

A concern with treatment of PDR eyes with anti-VEGF is the “crunch syndrome,” with development of tractional retinal detachments (TRDs). The DRCR Retina Network found that risk for progression of TRDs with anti-VEGF was not different from PRP. However, vitreous hemorrhage, macular-threatening TRDs, and fibrovascular proliferations were excluded from the DRCR and CLARITY studies and thus the results are not applicable for these patients. Another concern with anti-VEGF treatment is the risk of endophthalmitis in a diabetic patient. Endophthalmitis remains a real risk, albeit a rare one, and often can be successfully treated. Risk and benefit must be weighed for an individual patient. Rates of endophthalmitis in diabetic patients as a group appear similar to the rates of nondiabetic patients, such as AMD patients, undergoing intravitreal injections.
CONCLUSION
Anti-VEGF intravitreous injections are a good treatment choice for management of macular edema and PDR in patients who are more likely to be compliant. For DME, it offers the best chance of improving visual acuity. Focal laser is best reserved for nonfoveal DME associated with microaneurysms within circinate rings. For PDR, efficacy and safety of anti-VEGFs are as good as PRP as long as the patient can return for follow-up and treatment. Ancillary benefits include a lower risk for development of DME and vitreous hemorrhage, regression of the level of DR and preservation, at least initially, of the visual field. Certain patients, such as those at high risk of PDR with macular threatening TRDs, were not included in the clinical trials, and the results should not be extrapolated to include management of these patients. Overall, when selecting between PRP and anti-VEGF, the overarching consideration should be the patient’s ability to be compliant with follow-up visits. RP
REFERENCES
- Elman MJ, Aiello LP, Beck RW, et al; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.
- Elman MJ, Ayala A, Bressler NM, et al; Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381.
- Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022.
- Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID Studies. Ophthalmology. 2015;122(10):2044-2052.
- Michaelides M, Kaines A, Hamilton RD, et al. A Prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study): 12‐month data report 2. Ophthalmology. 2010;117(6):1078-1086.
- Wells JA, Glassman AR, et al.; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203.
- Wells JA, Glassman AR, Ayala AR, et al; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- Gross JG, Glassman AR, Jampol LM, et al; Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146.
- Gross JG, Glassman AR, Liu D, et al; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148.
- Sivaprasad S, Prevost AT, Vasconcelos JC, et al; CLARITY Study Group. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203.
- Gao X, Obeid A, Aderman CM, et al. Loss to follow-up after intravitreal anti-vascular endothelial growth factor injections in patients with diabetic macular edema. Ophthalmol Retina. 2019;3(3):230-236.
- Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology. 2018;125(9):1386-1392.
- Wubben TJ, Johnson MW; Anti-VEGF Treatment Interruption Study Group. Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am J Ophthalmol. 2019;204:13-18.
- Sacco RL, Wolf PA, Gorelick PB. Risk factors and their management for stroke prevention: outlook for 1999 and beyond. Neurology. 1999;53(7 Suppl 4):S15-S24.
- Bressler NM, Beaulieu WT, Glassman AR, et al; Diabetic Retinopathy Clinical Research Network. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257-269.
- Bressler NM, Beaulieu WT, Maguire MG, et al; Diabetic Retinopathy Clinical Research Network. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am J Ophthalmol. 2018;195:93-100.
- Baker CW, Glassman AR, Beaulieu WT, et al; DRCR Retina Network. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019 May 21;321(19):1880-1894.
- Bressler NM, Odia I, Maguire M, et al; DRCR Retina Network. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the Protocol T randomized clinical trial. JAMA Ophthalmol. 2019. [Epub ahead of print]
- Bressler SB, Odia I, Glassman AR, et al. Changes in diabetic retinopathy severity when treating diabetic macular edema with ranibizumab: DRCR.net Protocol I 5-year report. Retina. 2018;38(10):1896-1904.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: early treatment diabetic retinopathy study report number 1. Arch Ophthalmol. 1985;103:1796-1806.
- Bressler SB, Beaulieu WT, Glassman AR, et al; Diabetic Retinopathy Clinical Research Network. Photocoagulation versus ranibizumab for proliferative diabetic retinopathy: Should baseline characteristics affect choice of treatment? Retina. 2019. [Epub ahead of print]
- Sun JK, Glassman AR, Beaulieu WT, et al; Diabetic Retinopathy Clinical Research Network. Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87-95.








