Dr. Ehlers: As vitreoretinal specialists, we’re intimately familiar with the extent of the problem of diabetic eye disease. Of the more than 30 million people in the United States1 who have diabetes, approximately 25% develop retinopathy,2 and an estimated 10% are affected by diabetic macular edema (DME).3 We know, too, that diabetic eye disease is multifactorial, associated with not only upregulation of vascular endothelial growth factor (VEGF) but also higher levels of many other proinflammatory factors.4 Furthermore, it has been suggested that neurodegeneration plays a role in addition to vasculopathy5 and that both systemic and local inflammatory events contribute to the disease process (Figure 1).6,7

We see the complexities of diabetic eye disease play out every day in our practices. Some patients respond to therapy while others don’t. Some patients have significant vision loss without macular edema. Instead, they may exhibit ischemic changes, such as enlargement of the foveal avascular zone. We’ve also treated patients whose macular edema isn’t resolving, yet their vision is improving and vice versa. Ideally, we would like to have a better understanding of these differences and be able to predict which patients are most likely to progress to vision loss and which treatment modality is likely to be most effective. Imaging technologies, which have become more advanced and reliable, are helping to move us toward these goals. Imaging can reveal previously unseen changes in the eye that may be early indicators of disease and biomarkers for detection of subclinical retinal dysfunction.
Dr. Fortun: We’re in an interesting period, seeing a steady stream of papers exploring potential new biomarkers.
Optical Coherence Tomography (OCT): New Insights on the Gold Standard for DME Diagnosis and Monitoring
Dr. Ehlers: By measuring macular thickness and quantifying macular edema,8 OCT revolutionized the management of DME. Do you consider macular thickness to be a biomarker? Do you use macular thickness for deciding what treatment to use?
SriniVas R. Sadda, MD: Macular thickness doesn’t dictate which therapy I use first-line because I start with anti-VEGF therapy in the vast majority of DME patients. It may be that worse edema indicates a worse prognosis, but, in my experience, patients with significant edema often respond well to treatment.
Dr. Fortun: We can’t dogmatically assume that macular thickness of a certain extent is an indication that an eye will or will not respond to anti-VEGF treatment, but a post hoc subanalysis from the DRCR.net Protocol I study found baseline central subfield thickness (CST) to be the strongest predictor of anatomic outcome, and reduction in CST during the first treatment year was associated with better visual acuity outcomes.9 What likely matters more to most of us is the delta — the change in macular thickness we can produce with treatment.
Dr. Ehlers: Given the level of anatomic detail we obtain from OCT (Figure 2), what structures do you consider from a visual acuity standpoint in DME?

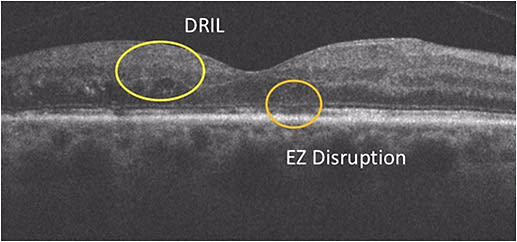
Dr. Sadda: Studies have linked disorganization of the retinal inner layers (DRIL) on OCT of eyes with DME to poorer visual acuity10 and less improvement in vision if it persists after resolution of the macular edema (Figure 3).11 In terms of the outer retinal bands, 2 areas also thought to have an impact on visual prognosis are the ellipsoid zone, which was formerly referred to as the photoreceptor inner segment/outer segment (IS/OS) junction, and the external limiting membrane (ELM).12,13
If the ellipsoid zone appears to be damaged but the ELM is intact, there may be an opportunity for vision recovery.13 We see that dynamic in other diseases as well, such as central serous chorioretinopathy (CSCR). However, when the ELM is also disrupted, it’s often a sign of irreversible damage with a poor visual prognosis, perhaps because it signifies irreversible Müller cell damage.
Dr. Ehlers: What about fluid characteristics — for example, intraretinal fluid vs subretinal fluid? With OCT, we learned, somewhat surprisingly, that some DME patients have subretinal fluid. From your reading center or clinical trial experience, or anecdotally from clinical practice, do fluid characteristics change your treatment approach or what result you expect to see?
Dr. Sadda: Subretinal fluid findings do not affect my initial decision in terms of therapy. I do pay attention to lipid exudates. Even in my patients whose retinal fluid is resolving, the lipids may continue to progress toward the fovea and enter the subretinal space, creating a poorer prognosis. For this reason, I like to achieve complete resolution of fluid before the lipids track further centrally. In these patients, the exudates disappear over time if the fluid is gone.
Dr. Ehlers: Do you modulate anything systemically if you see significant lipid?
Dr. Sadda: I generally don’t modulate the systemic therapy. I leave that to the internist. I do highlight my concern about the lipids in my communication with the internist and the patient.
Dr. Ehlers: Do you look for DRIL using OCT angiography (OCTA)? Do you find it correlates with DRIL seen on structural B-scans?
Dr. Sadda: Segmentation errors can be a challenge with OCTA, especially in diseases that affect the retinal morphology, so we have to be alert for that. Even though we think the deep capillary plexus is most severely impacted in diabetic patients, I’m concerned that I may not pick the segmentation correctly. Therefore, I think evaluating the entire retina may be the best way to assess DRIL on OCTA. Almost invariably, when I see DRIL on OCT, I see ischemia in that area on OCTA. I’m not as optimistic that a patient is going to return to perfect vision with that finding.
Dr. Fortun: For me, too, DRIL is more of a prognostic factor for function. It doesn’t really alter how I treat a patient.
Dr. Ehlers: As we pointed out, DRIL is associated with more severe retinopathy and poor visual acuity,15 but we don’t know how it’s linked to the pathogenesis. It’s not clear at this point whether it’s a definitive marker in the course of the disease.
One study has found that hyperreflective retinal spots (HRS) seen on OCT may correlate with functional data better than morphologic changes,16 perhaps representing clusters of activated microglial cells (Figure 4).14

Dr. Fortun: I see HRS on OCT, but I don’t necessarily look for them. I consider them together with other findings, such as DRIL, in my evaluation of disease severity. I don’t think they necessarily tell me I’m looking at a patient who’s going to need a particular treatment, but rather they suggest this is someone with worse disease, and I may need to start thinking about it as more of a heterogenic process.
Dr. Sadda: The meaning of the HRS, or the hyperreflective foci as they’re sometimes called, is not certain. For example, one study has found that if you correlate the larger ones against the color fundus photograph, they match up with lipid exudates;17 however, the data are inconclusive. But it’s not clear whether the really bright, large HRS are the same as the less bright, smaller HRS. It may be that hyperreflective foci in other diseases, such as CSCR, are in fact macrophages or activated microglia,18 but we don’t know if that also applies to DME.
Dr. Ehlers: To your point, it seems that in order to distinguish whether HRS are exudate or something else, it’s necessary to also evaluate them clinically or in a photo. Pigment migration in the outer retina has been associated with potential age-related macular degeneration disease progression.19
With HRS and DME, the situation seems somehow different. It’s more of a mystery exactly where the exudate is coming from and how to handle it. The HRS can appear in various locations, the inner retina, the outer retina, along vessels. A body of research that utilizes machine learning to analyze fluid components and characteristics is emerging.20
Dr. Sadda: Yes, interesting work has been done on fluid segmentation and evaluation of fluid burden. I think definitive answers about which morphologic parameters may predict treatment response and outcomes will come as artificial intelligence techniques become more sophisticated.
Dr. Ehlers: Currently, OCT is the primary method for deciding whether to treat a patient, but not necessarily which treatment to use. We’re focused, as we discussed previously, on the delta. In other words, we don’t consider changing a patient’s therapy until we’re not seeing the progress we want or expect.
Targeted Evaluation of Retinal Layers With OCT Angiography
Dr. Ehlers: OCTA seems to be the hottest topic in retinal imaging. Noninvasively, it allows separate evaluation of the superficial capillary plexus and the deep capillary plexus, the latter being where diabetic vascular changes primarily occur.21,22 When you acquire OCT scans, do you also acquire OCTA scans?
Dr. Fortun: I do, and I find OCTA shows me changes I wasn’t seeing before. While this may not change my treatment strategy, it changes how I stratify a patient’s risk. I also like OCTA for patient education. Often, if they don’t have vision loss, patients aren’t very concerned about diabetic eye disease. Showing them what is happening in their eye and explaining that the changes are a sign of things to come is helpful.
Dr. Sadda: We try to obtain OCTA images in as many patients as possible. It really doesn’t take additional time when we’re already taking OCT scans. As of now, we’re primarily using it as a research tool. That said, while we have a great deal to learn about how to use it clinically, OCTA is very much a ready-for-prime-time technology in some ways, particularly in assessing ischemia. It clearly does a much better job than fluorescein angiography (FA) in terms of quantifying macular nonperfusion.23 In fact, I no longer obtain dye-based angiography for assessment of macular perfusion.
OCTA may become a game changer with quantitative tracking of nonperfusion over time. It has been shown that OCTA retinal vascular perfusion density mapping correlates closely to clinical staging and may be an objective way to monitor progression of diabetic retinopathy.23,24
Dr. Ehlers: OCTA has shifted my threshold for ordering ultra-widefield FA (UWFA). I’ve been surprised by the amount of changes OCTA reveals that prompt me to order UWFA and the number of times it reveals subclinical neovascularization in the retinal periphery in the presence of those changes.
Dr. Sadda, can you explain more about the en face OCTA results slabs you assess in diabetic patients?
Dr. Sadda: I evaluate the full slab and then focus on the deep capillary plexus. As you said, most diabetic pathology resides in the deep plexus. Certainly, that’s where intraretinal microvascular abnormalities tend to occur. For example, microaneurysms can occur in the superficial capillary plexus or the deep capillary plexus, but they tend to appear in the deep layer. And those seem to be the most troublesome. I would venture to say OCTA is not 100% sensitive for detecting microaneurysms. One, it doesn’t pick up all of them, and, two, it doesn’t show which ones are leaking. Therefore, OCTA isn’t a useful tool for guiding focal laser treatment. In my opinion, it is useful for obtaining a quick assessment of where pathology is located. Spaide has highlighted the fact that the deep capillary plexus, particularly ischemia at that layer, may dictate where cystoid spaces accumulate in the retina.25 In other words, he believes the topography is very much dictated by alterations at the deep plexus level. I like to see what’s occurring in that area, although at this point, I’m mainly learning from the information rather than using it to change my management of patients.
Dr. Fortun: An important point to make about OCTA is that the information it provides is much more involved, much more granular than what we’ve had previously. In practice, we can’t simply look it over in the same way we scroll through 16 OCT line scans. So that raises the question: How do we use the data realistically? In the future, computing power may be used to provide a quantified overall picture.
Dr. Ehlers: While the field is still relatively young, it’s amazing how far the technology has come in terms of minimizing artifacts and motion correction, which were significant pitfalls at first. Opportunities we may have in the future for quantification include an algorithm referred to as “variable interscan time analysis” that would allow evaluation of relative blood flow speeds.26
Indeed, quantification with OCTA has become a broad area that multiple groups are studying now. We’ve mentioned two exciting areas so far, the potential for blood flow speed measurement and perfusion density mapping. But can we really use any of the quantification in practice right now, or are we still using qualitative review?
Dr. Fortun: I’m not exactly sure how to put the quantification software to use quite yet. I’m using my OCTA scans for more of a bird’s eye view. For example, does a patient’s retina have massive capillary dropout? In the future, I’m sure we’ll have easily obtained parameters from which we can build nomograms, for example, X number means this or that, but we’re probably a great deal of clinical trial work away from that goal.
Dr. Ehlers: Agreed. One of the aspects of OCTA I do enjoy right now, however, is longitudinal within the same patient. I don’t know for sure what to make of the information from patient to patient, but I feel it has potential for identifying a patient’s progression over time.
Dr. Sadda: I very much agree with both of you. While the quantitative tools are certainly interesting and seeing what’s happening over time in an individual patient is useful, at this stage, I’m using them primarily for research. I don’t know what to do with the information yet from a patient management standpoint. Most of the devices produce not only perfusion density values, but also something called vessel length density. We’re thinking vessel length density may be more useful in the long term. It may be more consistent between devices because it’s relatively resolution independent, which may be important. Progress is being made surrounding quantification, but we have a long way to go.
The better news is that almost all new clinical trials are utilizing OCTA, at the very least in substudies, so we’ll get real information on how to use it going forward.
Dr. Ehlers: The work being done by many of the reading center research groups to develop software that would aid comparative assessments between the various manufacturers’ devices will be crucial.
Faster Scan Speed of Swept-Source OCTA May Add Benefits
Dr. Ehlers: The potential of swept-source OCTA, with its faster scanning speed, promises increased tissue penetration, easier imaging through media opacities, and better visualization of the choroid.27 Do you see advantages to it compared with spectral-domain systems? Does swept-source OCTA have a role for you with your diabetic patients?
Dr. Fortun: My use of swept-source OCTA is more limited than my use of the spectral-domain devices, but I believe the higher resolution will help. We’ll know more as we use it more.
Dr. Ehlers: With most of the spectral-domain systems, I stick primarily with the 3×3 scan size because I feel that I lose detail when I use a larger scan size. In contrast, I feel I can go larger with the swept-source OCTA scan size.
Dr. Sadda: In my experience so far, I haven’t found swept-source OCTA to have any major advantages for vascular assessment of the macula. But, in my opinion, it can be of benefit for evaluating the choriocapillaris, certainly the choriocapillaris in the setting of pathology that significantly attenuates the signal.
I also agree that there are some potential benefits in wider-angle applications, which is an exciting opportunity for OCTA, at least in detection of abnormalities toward the arcades, particularly neovascularization. I think it will play a substantial role.
Dr. Ehlers: Yes, obviously, we’re still limited in how wide we can scan, but the macular vessel visualization with the spectral-domain systems is excellent, and the montaging of the periphery with swept-source produces pretty amazing images. The choriocapillaris is an interesting piece of the puzzle because in a great deal of the conditions we’re discussing, poor perfusion could be due to poor signal penetration or it could be actual nonperfusion in the choriocapillaris.
Ultra-Widefield Angiography’s Relevance in Diabetic Eye Disease
Dr. Ehlers: UWFA now enables visualization of nearly the entire retina in a single image,28 which may have significant implications in eyes with diabetic retinopathy and DME (Figure 5). Do you have this technology available in your clinics and in your satellite offices?


Dr. Fortun: We have UWFA in all but one of our clinics. We have really begun to realize its value, especially in our diabetic patient population.
Dr. Ehlers: When OCTA emerged, some people said FA is dead. What do you think?
Dr. Sadda: FA certainly isn’t dead yet, especially given OCTA’s current limitation of not providing vessel leakage information. Leakage, I think, is very relevant for identifying inflammation, for example. In addition, OCTA doesn’t have the ability to probe the far-peripheral vasculature, while UWFA has underscored the fact that extensive nonperfusion may exist in the periphery and it may affect prognosis and how we monitor and treat patients.
Dr. Ehlers: What’s your threshold for utilizing an UWFA?
Dr. Fortun: I don’t order UWFA in a fairly well-controlled diabetic patient with minimal retinopathy. For anyone with moderate diabetic eye disease, I obtain a baseline test. I may also obtain UWFA for a patient whose diabetes is poorly controlled and whose ophthalmoscopic exam indicates significant vascular attenuation. I think of this type of patient as a “featureless diabetic.” They’re a subset of patients in whom the disease is predominantly peripheral, not macular.

Dr. Ehlers: Dr. Sadda, you’ve published extensively on ultra-widefield imaging, including ischemia quantification and the challenges around distortion in the periphery. An interesting aspect of OCTA is that it seems to be elucidating for us that some of the peripheral ischemic changes may not be as clear as we thought. How do you approach that? Do you use the ischemic burden as any sort of biomarker as you make treatment choices; are you quantifying ischemia outside of a research setting?
Dr. Sadda: We feel we can quantify peripheral ischemia reliably using stereo projection–type tools that manage the challenge of transforming a 3-dimensional object into a 2-dimensional image. In a busy practice, it’s difficult to use quantification, so we do this primarily for research studies. We do think it has prognostic value, particularly in diabetes. UWFA has been used to show a correlation between peripheral ischemia and the risk of DME.29,30 The results of the DRCR.net Protocol AA study (peripheral diabetic retinopathy lesions on ultra-widefield fundus images and risk of diabetic retinopathy worsening over time) should shed more light in this area.31
In practice, I think it’s a bad sign when a patient has extensive peripheral nonperfusion or extensive peripheral lesions. But guidance from clinical trials about how this should affect patient management is currently not definitive. For example, in patients with retinal vein occlusion, the WAVE study was unable to confirm the results of a previous study in which targeted peripheral laser affected macular edema.32 However, researchers are getting more sophisticated with assessing nonperfusion with dye-based angiograms, which will help to provide clarity.
Dr. Ehlers: How do leakage patterns fit in with your treatment protocols?
Dr. Fortun: Vessel leakage and staining are important to me as a type of biomarker that I take into consideration along with all of the other biomarkers we’ve discussed. Taken together, they provide a sense of disease severity, and they prompt me to consider that more of an inflammatory process may be at work. When assessing response to treatment, I may think beyond whatever nonperfusion may exist and consider there may be a real role for suppressing inflammation.
Dr. Ehlers: It’s interesting because we see diabetic patients whose retinal vessels make them look like vasculitic patients. It seems to be the dominant leakage pattern versus more generalized leakage.
Returning to the topic of peripheral ischemia, Dr. Sadda, you spoke about some discordance in the literature as to the importance of peripheral ischemia as a biomarker. Do you think that UWFA will be our primary tool for measurement, or do you think we’ll be using OCTA more as it progresses?
Dr. Sadda: I don’t think it’s going to be either/or. We’ll most likely use them both. We have some caveats such as the difficulty of distinguishing nonperfusion after pharmacotherapy and, as was pointed out, difficulty distinguishing leakage and staining in a diabetic eye from leakage and staining in an eye with vasculitis or primary inflammatory disease. So, will we have a tool that can identify patients who presumably have more ischemia and also more vessel staining and leakage and therefore may have a prominent inflammatory component driving their diabetic pathology? This is an interesting idea to probe going forward. It will be important to include UWFA in studies evaluating diabetic retinopathy.
The Quest for Treatment-Guiding Biomarkers
Dr. Ehlers: My partner, Sunil Srivastava, MD, and I have been working on automating quantification of leakage.33 It’s a challenging area. As we evaluate our data sets, it seems that leakage activity may be one of the biggest drivers around some of the findings related to DME, more than microaneurysm burden and more than ischemia. It’s certainly the most dynamic. And as we look to diabetic retinopathy, how should we treat? On the DME end, we’ve become very image-driven in how we treat, but not so much on the diabetic retinopathy end. What, for example, would be a 2-step improvement? How are you going about that?
Dr. Fortun: You raise an interesting point. What should we do with a patient who has no DME, but we see these features we’ve been discussing that we think point to worsening diabetic retinopathy, which we now have drugs approved to treat? Who should we treat? What’s the threshold to treat? The patient with noncentral DME, for example, we typically wouldn’t treat; our focus is on the macula. But this is a patient who has enough capillary damage to lead to leakage. So, should treatment have been started earlier? Should we utilize medications in an attempt to at least slow the progression of changes seen on widefield imaging even if they’re not affecting vision?
Dr. Ehlers: It may be the computing power we mentioned that will allow us to have the quantitative information we need, whether it’s within the angiogram or the OCT, to guide how we think about change analysis and improve profiling of which treatment is most appropriate for which patient.
What are your thoughts on whether we’ll eventually be able to use biomarkers imaged with OCT, OCTA, and/or UWFA to make confident treatment decisions? Certainly, many opportunities and possibilities exist, but none so far has the backing of definitive data that allows us to determine the pathogenesis of diabetic eye disease in an individual.
Dr. Sadda: I predict imaging biomarkers will be useful and important, and artificial intelligence and deep learning will help us determine how to integrate all of the information and how best to use it to guide patient management. What we can do right now is advocate for incorporating these new diagnostic technologies into clinical trials to expand our understanding of their utility. That will lead us toward better tools for individualizing our therapies.
Dr. Ehlers: Yes, we’re in an exciting time with new diagnostics and new treatments. Clinical trials involving both aspects will be crucial for helping us choose the best course of action for individual patients.
Dr. Fortun: Biomarkers are going to be important in the context of diabetic retinopathy and DME, which are the end of the disease road, so to speak. They’re also going to be important for guiding the treatment of asymptomatic patients. Again, as with every other aspect of diabetes, prevention is key. What we’re really seeking is the ability to treat patients based on biomarkers before they progress to proliferative diabetic retinopathy and DME, to know exactly what we’re targeting among the many factors that are involved.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Centers for Disease Control and Prevention website. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html . Updated February 24, 2018. Accessed July 19, 2019.
- Keenum Z, McGwin G Jr, Witherspoon CD, Haller JA, Clark ME, Owsley C. Patients’ adherence to recommended follow-up eye care after diabetic retinopathy screening in a publicly funded county clinic and factors associated with follow-up eye care use. JAMA Ophthalmol. 2016;134(11):1221-1228.
- Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010;26(7):1587-1597.
- Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443-2453.
- Midena E, Pilotto E. Emerging insights into pathogenesis. Dev Ophthalmol. 2017;60:16-27.
- Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol. 2012;19(1):52-59.
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343-358.
- Bhagat N, Zarbin MA. Lim JI, Tripathy K, Leng T. Diabetic macular edema. American Academy of Ophthalmology website. https://eyewiki.aao.org/Diabetic_Macular_Edema . Updated December 8. 2018. Accessed August 8, 2019.
- Bressler SB, Qin H, Beck RW, et al; Diabetic Retinopathy Clinical Research Network. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153-1161.
- Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316.
- Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133(7):820-825.
- Ito S, Miyamoto N, Ishida K, Kurimoto Y. Association between external limiting membrane status and visual acuity in diabetic macular oedema. Br J Ophthalmol. 2013;97(2):228-232.
- Muftuoglu IK, Unsal E, Ozturker ZK. Restoration of photoreceptors in eyes with diabetic macular edema. Eur J Ophthalmol. 2017;27(5):585-590.
- Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273.
- Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202-208.
- Vujosevic S, Berton M, Bini S, Casciano M, Cavarzeran F, Midena E. Hyperreflective retinal spots and visual function after anti-vascular endothelial growth factor treatment in center-involving diabetic macular edema. Retina. 2016;36(7):1298-1308.
- Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C; Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914-920.
- Turgut B, Yildirim H. The causes of hyperreflective dots in optical coherence tomography excluding diabetic macular edema and retinal venous occlusion. Open Ophthalmol J. 2015;9:36-40.
- Christenbury JG, Folgar FA, O’Connell RV, Chiu SJ, Farsiu S, Toth CA; Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group. Progression of intermediate age-related macular degeneration with proliferation and innerretinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038-1045.
- Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1-29.
- Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016;123(11):2368-2375.
- Moon BG, Um T, Lee J, Yoon YH. Correlation between deep capillary plexus perfusion and long-term photoreceptor recovery after diabetic macular edema treatment. Ophthalmol Retina. 2018;2(3):235-243.
- Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(4):367-373.
- Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353-2363.
- Spaide RF. Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 2015;160(6):1200-1210.
- Ploner SB, Moult EM, Choi W, et al. Toward quantitative optical coherence tomography angiography: visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina. 2016;36(suppl 1):118S-126S.
- La Mantia A, Kurt RA, Mejor S, et al. Comparing fundus fluorescein angiography and swept-source optical coherence angiography in the evaluation of diabetic macular ischemia. Retina. 2019;39(5):926-937.
- Kim EL, Moshfeghi AA. Wide-field imaging of retinal diseases. US Ophthalmic Review. 2015;8(2):125-131.
- Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698.
- Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1-32.
- Diabetic Retinopathy Clinical Research Network. Peripheral diabetic retinopathy (DR) lesions on ultrawide-field fundus images and risk of DR worsening over time. DRCRnet website. http://publicfiles.jaeb.org/Peripheral_DR_lesions_and_Risk_of_DR_ProgressionV4_0_7_21_17.pdf . Published July 21, 2017. Accessed August 8, 2019.
- Wykoff CC, Ou WC, Wang R, et al; WAVE Study Group. Peripheral laser for recalcitrant macular edema owing to retinal vein occlusion: the WAVE trial. Ophthalmology. 2017;124(6):919-921.
- Ehlers JP, Wang K, Vasanji A, Hu M, Srivastava SK. Automated quantitative characterisation of retinal vascular leakage and microaneurysms in ultra-widefield fluorescein angiography. Br J Ophthalmol. 2017;101(6):696-699.








