Pathologic myopia is one of the leading causes of visual impairment worldwide, including the leading cause of blindness in East Asia.1 High myopia is generally defined as a spherical equivalent of ≥-6.0 diopters or an axial length ≥26.0 mm2. Furthermore, a recent review article estimated the prevalence of high myopia will increase from 2.7% of the world’s population in 2000 to 9.8% by 2050.3 Pathologic or degenerative myopia is defined as the presence of structural changes due to axial elongation in eyes with high myopia.4 Numerous vision-threatening conditions are known to be more prevalent in eyes with pathologic myopia including retinal detachment, myopic retinoschisis, macular holes, choroidal neovascularization, and chorioretinal atrophy. This article summarizes the diagnosis and management of posterior-segment complications from pathologic myopia.
POSTERIOR STAPHYLOMA
Posterior staphyloma was first described by Scarpa in 1801.5 More recently, Spaide defined the entity as a circumscribed outpouching of the ocular wall with a radius of curvature that is less than the surrounding eye wall curvature (Figure 1).6 A posterior staphyloma is a hallmark of pathologic myopia, but it can also occur in the absence of high myopia. The development of posterior staphyloma increases with both age and axial length.7,8 It can occur isolated or in combination around the optic nerve, in the macula, or anywhere in the posterior segment. Multiple tissues have been implicated in the development of a posterior staphyloma with the sclera and choroid considered most prominent, as they suffer the most relative thinning. Diagnosis is most commonly made by dilated fundus exam, optical coherence tomography (OCT), and/or B-mode echography.9 There is no approved treatment to alter the natural history of posterior staphyloma, but scleral collagen cross-linking and scleral regeneration have been investigated. Curtin’s posterior staphyloma categorization system of 10 types10 has been recently simplified by Ohno-Matsui, who classified posterior staphyloma into 6 types: wide macular, narrow macular, peripapillary, nasal, inferior, and other configurations.7

DOME-SHAPED MACULA
Dome-shaped macula is an anterior convex deviation of the macular sclera, choroid, and retina within a posterior staphyloma.11 The 3 morphologies are described as a horizontally oriented, vertically oriented, and rounded,12 which may be secondary to a relative thickening of the sclera compared to surrounding areas.13 Dome-shaped macula is commonly associated with a pigment epithelium detachment (PED) and subretinal fluid (SRF), whereas pathogenesis of PED may be secondary to a relative thickening of the submacular choroid.11 A dome-shaped macula bulge height >400 micrometers has been associated with PED, retinal pigment epithelium atrophy, and decreased visual acuity.14
To date, there is a lack of consensus for treating dome-shaped macula associated subfoveal fluid. Spontaneous resolution of subfoveal fluid has been reported,15 and it may mask the results of potential treatments, such as photodynamic therapy, laser photocoagulation, anti-VEGF intravitreal injections, and steroids.8
CHORIORETINAL ATROPHY
Atrophic myopic maculopathy is the myopic equivalent of atrophic age-related macular degeneration (AMD)8. Progressive chorioretinal atrophy is associated with age, axial length, staphyloma, myopic choroidal neovascularization (CNV), and myopic traction maculopathy (MTM).16 Myopic maculopathy (Figure 2) can appear in different patterns. An international group of high myopia experts recently created a simplified classification of pathologic myopia (META-PM): no myopic lesions (category 0), tessellated fundus (category 1), diffuse chorioretinal atrophy (category 2), patchy chorioretinal atrophy (category 3), and macular atrophy (category 4).17 Up to 3 “plus signs,” which can develop from or coexist with any of the above categories, can be added if there is the presence of CNV, lacquer cracks, and Fuch’s spot.1 A newer classification system based on atrophy, traction, and neovascularization may gain wider acceptance.8

Although patients with even extreme chorioretinal thinning can have good vision,18 progressive macular thinning will cause a definite decline in vision. Currently, there is no pharmacologic treatment available. In the future, analogous treatments for atrophic AMD may also prove useful for myopic chorioretinal atrophy.
NEOVASCULAR MYOPIC MACULOPATHY
Neovascular myopic maculopathy is a proliferation of a choroidal neovascularization complex in myopic patients. Roughly 5% to 11% of patients with pathologic myopia will develop myopic CNV, and 35% of those with myopic CNV will develop bilateral involvement.19 Risk factors for the development of CNV include retinal atrophy, lacquer cracks, choroidal thinning, and delayed choroidal filling on angiography. There are several proposed mechanisms for the development of myopic CNV. Progressive axial length elongation may disrupt Bruch’s membrane, leading to increased exposure of VEGF, which can stimulate CNV development.20 Thinner myopic choroids lead to low vascular exudation, revealing subtle leakage on fluorescein angiography21 or optical coherence tomography angiography (OCTA).22
Anti-VEGF injections are highly effective for the treatment of myopic CNV. Both ranibizumab (Lucentis; Genentech) and aflibercept (Eylea; Regeneron) have been approved for myopic CNV23,24, but intravitreal bevacizumab (Avastin; Genentech) has shown efficacy in nonrandomized clinical trials and is in common use off-label in clinical practice.25 Furthermore, anti-VEGF treatment more frequently leads to regression of myopic CNV, allowing cessation, when compared to similar treatment for neovascular AMD.
MYOPIC TRACTION MACULOPATHY
Myopic traction maculopathy encompasses vitreomacular traction (VMT), foveoschisis, macular hole, and macular hole retinal detachment in myopic patients. In elongated eyes, especially those with a posterior staphyloma, the relative noncompliance of the inner retina (especially the ILM) leads to stretching in an anterior-posterior direction as the outer retina follows the contour of the sclera.26 Optical coherence tomography is essential to establish diagnosis and monitor changes over time (Figure 3). Myopic foveoschisis is a separation of the retinal layers that remain connected by Muller cells.27 There is no medical treatment for this entity. Therefore, surgical intervention may be considered when there is progressive loss of visual acuity with corresponding anatomic changes.8 Myopic foveoschisis progression may lead to foveal detachment and macular hole, which carry a worse functional prognosis, and can lead to retinal detachment.28
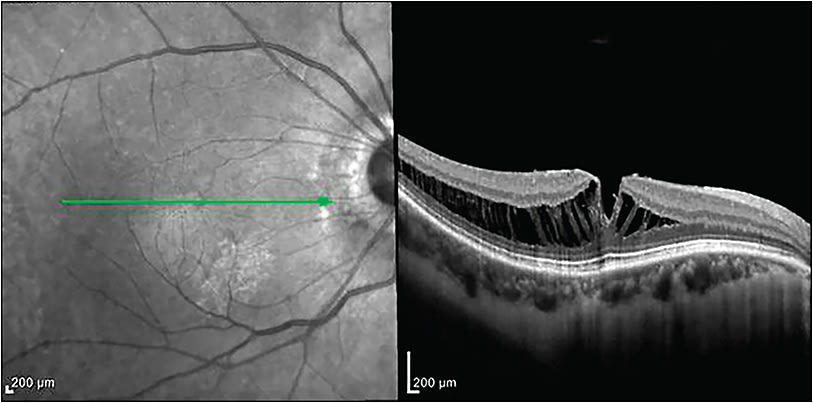
Although surgery is the primary treatment for vision loss related to myopic foveoschisis, the role of pars plana vitrectomy (PPV) with posterior hyaloid dissection and internal limiting membrane (ILM) and/or epiretinal membrane (ERM) peeling is controversial. A recent meta-analysis indicated better anatomic results, but no statistically significant difference was seen in patients who had a vitrectomy with or without ILM peeling.29 A fovea-sparing technique may be employed for myopic foveoschisis, where some of the ILM is left in the center of the macula to protect Muller cells and reduce risk of iatrogenic macular hole development.30
In the treatment of myopic macular holes and macular hole retinal detachments (Figure 1), most surgeons will perform PPV with hyaloid dissection and ILM/ERM peeling over the entire macula.8 Like foveoschisis treatment, some surgeons do prefer a fovea sparing approach, leaving an ILM shield over the macula to protect the fovea.31 Peeling visualization can be aided by using dyes to stain the ILM and/or ERM, and macular flattening can be improved with perfluorocarbon liquid. In most cases, placement of gas tamponade with postoperative face-down positioning is recommended after macular hole surgery.8 Macular hole retinal detachment carries a poor prognosis and can be difficult to repair. Numerous techniques are described to aid in primary and secondary closure of myopic macular holes including ILM flaps, ILM translocation, and even localized retinal transplantation. Alternatively, PPV with ILM/ERM peeling can be combined with macular buckling has been advocated to flatten the posterior staphyloma and facilitate hole closure.32
OTHER POTENTIAL MYOPIC COMPLICATIONS
Peripheral retinal degeneration occurs more frequently in highly myopic eyes. Overall, the risk of retinal detachment is nearly 20 times higher in myopic eyes than in emmetropic eyes.33 Rhegmatogenous retinal detachment (RRDs) after cataract surgery is uncommon, but they are twice as likely in highly myopic eyes.34 In addition, posterior vitreous detachment occurs at a younger age in high myopia, which may contribute to the increased prevalence of RRDs.35
Progressive axial length elongation may lead to expansion of the optic nerve head and thinning of the lamina cribosa.36,37 High myopia is associated with normal tension glaucoma, denoting high myopes’ susceptibility to increased intraocular pressure.38 With increasing mechanical stress, peripapillary cavitation, and optic pit may also occur.39,40
Finally, the introduction of widefield photography has led to the discovery of peripheral vascular perfusion abnormalities in highly myopic eyes. Decreased perfusion can lead to capillary telangiectasias, microaneurysms, and nonperfusion.41
CONCLUSION
Myopic visual impairment can occur through a number of mechanisms, including posterior staphyloma, dome-shaped macula, chorioretinal atrophy, neovascular myopic maculopathy, myopic traction maculopathy, retinal detachment, and optic nerve damage. Future advances in imaging technology and treatment modalities may lead to greater disease pathology understanding and improved patient outcomes. Myopia’s vision threatening complications and increasing prevalence necessitates thorough diagnosis and management by the retinal physician. RP
REFERENCES
- Chan NS, Teo K, Cheung CM. Epidemiology and diagnosis of myopic choroidal neovascularization in Asia. Eye Contact Lens. 2016;42(1):48-55.
- Ohno-Matsui K. What is the fundamental nature of pathologic myopia? Retina. 2017;37(6):1043-1048.
- Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042.
- Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739-1748.
- Scarpa A. [Essay on observations and experiences with the main eye diseases.] Pavia: Baldassare Comino; 1801:278.
- Spaide RF. Staphyloma: part 1. In: RF Spaide, K Ohno-Matsui, LA YAnnuzzi, eds. Pathologic Myopia. New York: Springer-Verlag; 2014:167-185.
- Ohno-Matsui K. Pathologic myopia. Asia Pac J Ophthalmol. 2016;5(6):415-423.
- Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80-115.
- Ohno-Matsui K, Jonas JB. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res. 2019;70:99-109.
- Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1977;75:67-86.
- Gaucher D, Erginay A, Lecleire-Collet A, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol. 2008;145(5):909-914.
- Caillaux V, Gaucher D, Gualino V, Massin P, Tadayoni R, Gaudric A. Morphologic characterization of dome-shaped macula in myopic eyes with serous macular detachment. Am J Ophthalmol. 2013;156(5):958-967.e1.
- Ohsugi H, Ikuno Y, Oshima K, Tabuchi H. 3-D choroidal thickness maps from EDI-OCT in highly myopic eyes. Optom Vis Sci. 2013;90(6):599-606.
- Fajardo Sanchez J, Chau Ramos CE, Roca Fernandez JA, Urcelay Segura JL. Clinical, fundoscopic, tomographic and angiographic characteristics of dome shaped macula classified by bulge height. Arch Soc Esp Oftalmol. 2017;92:458-463.
- Tamura N, Sakai T, Tsuneoka H. Spontaneous resolution of foveal detachment in dome-shaped macula observed by spectral domain optical coherence tomography. Clin Ophthalmol. 2014;8:83-86.
- Barteselli G, Lee SN, El-Emam S, et al. Macular choroidal volume variations in highly myopic eyes with myopic traction maculopathy and choroidal neovascularization. Retina. 2014;34(5):880-889.
- Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877-883.e7.
- Pang CE, Sarraf D, Freund KB. Extreme choroidal thinning in high myopia. Retina. 2015;35(3):407-415.
- Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018;63:92-106.
- Ohno-Matsui K, Jonas JB, Spaide RF. Macular Bruch Membrane Holes in Highly Myopic Patchy Chorioretinal Atrophy. Am J Ophthalmol. 2016;166:22-28.
- Flores-Moreno I, Ruiz-Medrano J, Duker JS, Ruiz-Moreno JM. The relationship between retinal and choroidal thickness and visual acuity in highly myopic eyes. Br J Ophthalmol. 2014;97(8):143-144.
- Bruyere E, Miere A, Cohen SY, et al. Neovascularization secondary to high myopia imaged by optical coherence tomography angiography. Retina. 2017;37(11):2095-2101.
- Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology. 2015;122(6):1220-1227.
- Wolf S, Balciuniene VJ, Laganovska G, et al; RADIANCE Study Group. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682-692.e2.
- Hashemi S, Faramarzi MA, Ghasemi Falavarjani K, Abdollahi M. Bevacizumab for choroidal neovascularization secondary to age-related macular degeneration and pathological myopia. Expert Opin Biol Ther. 2014;14(12):1837-1848.
- VanderBeek BL, Johnson MW. The diversity of traction mechanisms in myopic traction maculopathy. Am J Ophthalmol. 2012;153(1):93-102.
- Phillips CI. Retinal detachment at the posterior pole. Br J Ophthalmol. 1958;42(12):749-753.
- Sayanagi K, Ikuno Y, Tano Y. Tractional internal limiting membrane detachment in highly myopic eyes. Am J Ophthalmol. 2006;142(5):850-852.
- Meng B, Zhao L, Yin Y, et al. Internal limiting membrane peeling and gas tamponade for myopic foveoschisis: a systematic review and meta-analysis. BMC Ophthalmol. 2017;17(1):166.
- Ho TC, Yang CM, Huang JS, et al. Long-term outcome of foveolar internal limiting membrane nonpeeling for myopic traction maculopathy. Retina. 2014;34(9):1833-1840.
- Shimada N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol. 2012;154(4):693-701.
- Ohsugi H, Ikuno Y, Matsuba S, et al. Morphologic characteristics of macular hole and macular hole retinal detachment associated with extreme myopia. Retina. 2019;39(7):1312-1318.
- Pierro L, Camesasca FI, Mischi M, Brancato R. Peripheral retinal changes and axial myopia. Retina. 1992;12(1):12-17.
- Neuhann IM, Neuhann TF, Heimann H, Schmickler S, Gerl RH, Foerster MH. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34(10):1644-1657.
- Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100(9):1384-1388.
- Jonas JB, Jonas SB, Jonas RA, Holbach L, Panda-Jonas S. Histology of the parapapillary region in high myopia. Am J Ophthalmol. 2011;152(6):1021-1029.
- Wang Y, Xu L, Zhang L, Yang H, Ma Y, Jonas JB. Optic disc size in a population based study in northern China: the Beijing Eye Study. Br J Ophthalmol. 2006;90(3):353-356.
- Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007;114(2):216-220.
- Yeh SI, Chang WC, Wu CH, et al. Characteristics of peripapillary choroidal cavitation detected by optical coherence tomography. Ophthalmology. 2013;120(3):544-552.
- Freund KB, Ciardella AP, Yannuzzi LA, et al. Peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2003;121(2):197-204.
- Kaneko Y, Moriyama M, Hirahara S, Ogura Y, Ohno-Matsui K. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55(3):1432-1439.








