Increased knowledge of the genetic defects associated with age-related macular degeneration (AMD) have given rise to several potential therapeutic interventions involving the various components of the complement pathway. High-dose antioxidant oral vitamins were first used in clinical trials and showed a mild protective effect in reducing disease progression to neovascular AMD. Unfortunately, this protective effect did not influence the formation or growth of geographic atrophy (GA). Other therapies have been developed and evaluated in clinical trials to mitigate the pathogenetic pathways involved in AMD. These include the use of anti-inflammatory drugs, drugs to inhibit various pathways in the complement cascade (these include gene therapies), and stem cell strategies. To date, there are no approved treatments for GA. An alternative approach is to use a cytoprotective or neuroprotective drug, which may allow intervention at a common point in the pathogenesis to stabilize and protect the retinal pigment epithelium (RPE) and photoreceptors, hence making these cells more resistant to the pathogenetic mechanisms involved in GA.
BRIMONIDINE
Brimonidine is a highly selective alpha2-adrenergic receptor agonist that is well known as an anti-glaucoma agent that lowers IOP, but it also has cytoprotective and neuroprotective properties. The important protective effect of this drug has been observed in studies of cultured cells and in animal models of optic nerve and retinal damage. Importantly, the drug has reduced the toxic effects of hydroquinone in RPE cells and human retinal Muller cells.1 And, even after topical administration, photoreceptor damage from blue light exposure is reduced.2 Systemic or topical brimonidine has promoted the survival of retinal ganglion cells in multiple rodent models of optic nerve injury, including acute retinal ischemia,3-6 excitotoxic retinal injury,7 chronic ocular hypertension,8,9 and optic nerve crush injury models.10,11 In a nonhuman primate model of GA due to focal light exposure, the Brimonidine Drug Delivery System (Brimo DDS; Allergan), an intravitreal implant consisting of brimonidine in a poly-(D,L-lactide) biodegradable polymer matrix, was shown to protect both RPE and photoreceptors from blue-light–induced cytotoxicity. This effect was more readily observed with more aggressive lesions.12
William R. Freeman, MD, is the distinguished professor and director of the Jacobs Retina Center and the vice chair of the department of ophthalmology at the Shiley Eye Center of the University of California San Diego in La Jolla, California. Dr. Freeman reports consultancy to Alcon, Allergan, and Genentech and equity interest in Spinnaker Biosciences and Nanovision. Reach him at wrfreeman@ucsd.edu.
Clinical evidence that topical brimonidine is neuroprotective has been shown in studies of glaucoma,13 and brimonidine has been suggested to be useful in AMD patients who had laser-treated choroidal neovascularization14 as well as in some studies of retinitis pigmentosa,15 diabetic retinopathy,16 and acute nonarteritic anterior ischemic optic neuropathy.17 In a large study, the Low-Pressure Glaucoma Treatment Study, topical brimonidine showed greater reduction in visual-field progression than with timolol despite a similar pressure-lowering effect.13 This neuroprotective effect seems to improve survival and function of neurons, probably due to stimulation of the widely expressed alpha2-adrenergic receptor, which is present throughout the neurosensory retina. Stimulation of this receptor by brimonidine has been suggested to activate cell-survival signaling pathways, upregulate neurotrophic factors, and interfere with excitotoxic signaling.11,18-20
To provide a higher concentration of brimonidine in the retina and related tissues, Allergan has incorporated it into a polymer delivery system similar to that used for its Ozurdex dexamethasone intravitreal implant, but smaller (25 gauge vs 22 gauge for Ozurdex). This system is proven to permit release of drugs that are noncovalently bound to the polymer, which dissolves into nontoxic sugars within the vitreous, releasing the active ingredient.
Given its demonstrated cytoprotective and neuroprotective effects, brimonidine is currently being investigated for the treatment of GA secondary to AMD. The goal of cytoprotection and neuroprotection in GA is to reduce the rate of GA progression and stabilize loss of visual function by protecting the RPE and overlying photoreceptors, increasing their resistance to injury, and preventing or delaying cell death.21
Pharmacokinetic considerations suggest that effective drug levels in the retina and RPE must be maintained to impact GA. Topical brimonidine (0.2%) shows some evidence of therapeutic concentrations in the retina in pseudophakic eyes; however, levels may not be adequate for full cytoprotection or neuroprotection.22 Intravitreal administration yields therapeutic retinal levels, but the compound has a 10-hour half-life,23 which would make frequent intraocular injections necessary. Animal studies have suggested that a polymer insert with the drug (Brimo DDS) delivers therapeutic levels highest in the retina with lower levels in the vitreous, and even lower in the aqueous. In nonhuman primates, pharmacokinetic studies suggested that therapeutic levels would be present in the macula with administration every 3 months. The implant was tested in primates in a blue-light model of retinal damage, and a therapeutic effect for up to 15 weeks was observed.12 In clinical studies, there were 2 generations of Brimo DDS, with the first generation containing a dose of 132 µg or 264 µg and the second generation using a 400 µg brimonidine dose. The implant is administered via intravitreal injection using a 25-gauge needle and a proprietary applicator system.
BRIMO DDS DEVELOPMENT PROGRAM FOR GEOGRAPHIC ATROPHY
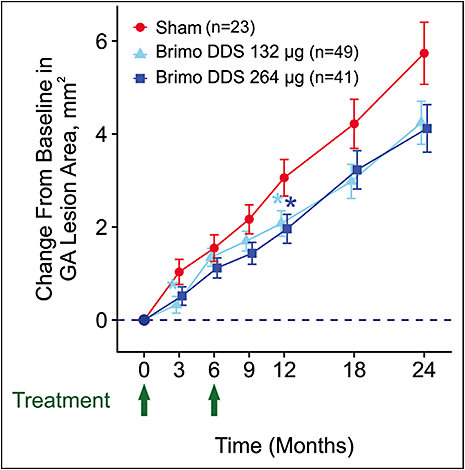
A phase 2a clinical trial evaluated safety and efficacy of Brimo DDS generation 1 containing either 132 μg or 264 μg brimonidine on retinal structure and visual function in patients with GA secondary to AMD. In this multicenter, double-masked, 24-month study (NCT00658619), for inclusion, patients had to have at least 1 site of GA with a lesion area of 0.75 to 12 disc areas (2.02-32.28 mm2) that resided at least in part within 1,500 µm of the foveal center.24 Patients were randomized to study eye treatment (day 1; month 6 retreatment) with Brimo DDS 132 µg (n=49), Brimo DDS 264 μg (n=41), or sham procedure (n=23). The primary outcome measure was change in the GA lesion area from baseline to month 12 (Figure 1). Mean GA lesion size at baseline was approximately 11 mm2. Mean GA lesion area growth was consistently smaller with Brimo DDS 132 µg and 264 µg than sham with between-group differences that were significant (P=.032 for Brimo DDS 132 µg and P=.028 for Brimo DDS 264 µg) at month 3. The effects of Brimo DDS on GA lesion growth were most apparent in patients with larger lesions at baseline. Change in lesion area and radius were significantly reduced with both Brimo DDS 132 µg and 264 µg vs sham at month 12 in patients with baseline lesion area ≥6 mm2 (two-thirds of patients; P≤.050; B. D. Kuppermann, MD, et al, unpublished data, 2019). Brimo DDS demonstrated a favorable safety profile. Treatment-related adverse events were usually injection procedure related and included redness at the injection site and floaters.
A larger multicenter, randomized, double-masked, controlled phase 2b study (BEACON; NCT02087085) evaluated a more potent formulation of the implant, a second-generation Brimo DDS 400 µg in a tartrate form, administered at 3-month intervals compared with sham procedure in patients with GA secondary to AMD. Eligible eyes had GA areas between 1.25 mm2 and 18 mm2. The primary endpoint was the change in GA lesion area from baseline. Fundus autofluorescence showed a 7% reduction in GA area growth from baseline at month 24 and a statistically significant 11% reduction at month 30. The BEACON study was stopped at interim analysis because of a slow GA lesion progression rate (~1.6 mm2/year) in the enrolled population, which had a mean baseline GA lesion area of approximately 5 mm2. Nevertheless, the implant significantly reduced lesion growth at the final month 30 time point. The results of both the phase 2a and 2b studies provide evidence of safety and efficacy, and 2 definitive phase 3 randomized, prospective multicenter studies of the second-generation Brimo DDS at 200 µg and 400 µg doses (IMAGINE and ENVISION) are planned to commence shortly.
At the moment, there are no proven therapies for GA. There have been several failures of anti-complement therapy, although more studies are under way. Brimo DDS shows promise of efficacy and may be the first long-acting therapy to slow the progression of GA secondary to AMD. RP
REFERENCES
- Ramirez C, Cáceres-del-Carpio J, Chu J, et al. Brimonidine can prevent in vitro hydroquinone damage on retinal pigment epithelium cells and retinal Müller cells. J Ocul Pharmacol Ther. 2016;32(2):102-108.
- Ortin-Martinez A, Valiente-Soriano FJ, Garcia-Ayuso D, et al. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS One. 2014;9(12):e113798.
- Lafuente MP, Villegas-Perez MP, Sobrado-Calvo P, Garcia-Aviles A, Miralles de Imperial J, Vidal-Sanz M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2001;42(2):2074-2084.
- Vidal-Sanz M, Lafuente MP, Mayor S, de Imperial JM, Villegas-Perez MP. Retinal ganglion cell death induced by retinal ischemia. neuroprotective effects of two alpha-2 agonists. Surv Ophthalmol. 2001;45 Suppl 3:S261-S267.
- Lai RK, Chun T, Hasson D, Lee S, Mehrbod F, Wheeler L. Alpha-2 adrenoceptor agonist protects retinal function after acute retinal ischemic injury in the rat. Vis Neurosci. 2002;19(2):175-185.
- Donello JE, Padillo EU, Webster ML, Wheeler LA, Gil DW. Alpha(2)-adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exper Ther. 2001;296(1):216-223.
- Galindo-Romero C, Harun-Or-Rashid M, Jimenez-Lopez M, Vidal-Sanz M, Agudo-Barriuso M, Hallbook F. Neuroprotection by alpha2-adrenergic receptor stimulation after excitotoxic retinal injury: a study of the total population of retinal ganglion cells and their distribution in the chicken retina. PLoS one. 2016;11(9):e0161862.
- WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42(12):2849-2855.
- Hernandez M, Urcola JH, Vecino E. Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp Eye Res. 2008;86(5):798-806.
- Yoles E, Wheeler LA, Schwartz M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci. 1999;40(1):65-73.
- Saylor M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch Ophthalmol. 2009;127(4):402-406.
- Rajagopalan L, Ghosn C, Tamhane M, et al. Cyto-/neuro-protective effects of brimonidine drug delivery system (DDS) in a nonhuman primate progressive retinal degeneration model of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Poster presented at: Association for Research in Vision and Ophthalmology; Vancouver, Canada; April 30, 2019.
- Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151(4):671-681.
- Ferencz JR, Gilady G, Harel O, Belkin M, Assia EI. Topical brimonidine reduces collateral damage caused by laser photocoagulation for choroidal neovascularization. Graefes Arch Clinical Exper Ophthalmol. 2005;243(9):877-880.
- Merin S, Obolensky A, Farber MD, Chowers I. A pilot study of topical treatment with an alpha2-agonist in patients with retinal dystrophies. J Ocul Pharmacol Ther. 2008;24(1):80-86.
- Mondal LK, Baidya KP, Bhattacharya B, Chatterjee PR, Bhaduri G. The efficacy of topical administration of brimonidine to reduce ischaemia in the very early stage of diabetic retinopathy in good controlled type-2 diabetes mellitus. J Indian Med Assoc. 2004;102(12):724-725, 729.
- Wilhelm G, Lüdtke H, Wilhelm H; BRAION Study Group. Efficacy and tolerability of 0.2% brimonidine tartrate for the treatment of acute non-arteritic anterior ischemic optic neuropathy (NAION): a 3-month, double-masked, randomised, placebo-controlled trial. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):551-558.
- Gao H, Qiao X, Cantor LB, WuDunn D. Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch Ophthalmol. 2002;120(6):797-803.
- Peng M, Li Y, Luo Z, Liu C, Laties AM, Wen R. Alpha2-adrenergic agonists selectively activate extracellular signal-regulated kinases in Muller cells in vivo. Invest Ophthalmol Vis Sci. 1998;39(9):1721-1726.
- Wheeler L, WoldeMussie E, Lai R. Role of alpha-2 agonists in neuroprotection. Surv Ophthalmol. 2003;48 Suppl 1:S47-S51.
- Danis RP, Lavine JA, Domalpally A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin Ophthalmol. 2015;9:2159-2174.
- Kent AR, Nussdorf JD, David R, Tyson F, Small D, Fellows D. Vitreous concentration of topically applied brimonidine tartrate 0.2%. Ophthalmology. 2001;108(4):784-787.
- Shen J, Durairaj C, Lin T, Liu Y, Burke J. Ocular pharmacokinetics of intravitreally administered brimonidine and dexamethasone in animal models with and without blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2014;55(2):1056-1066.
- Freeman WR, Bandello F, Souied E, et al. Brimonidine DDS safety and efficacy in patients with geographic atrophy secondary to age-related macular degeneration. Presented at: Annual meeting of the Association for Research in Vision and Ophthalmology; Vancouver, Canada; April 28, 2019.








