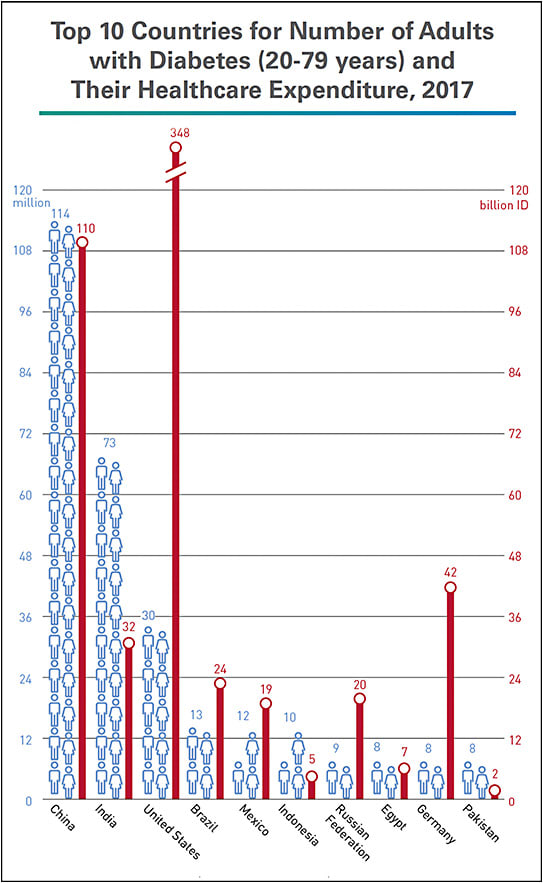
According to the International Diabetes Federation (IDF), $727 billion — 12% of total global health expenditure — is spent on diabetes.1 For the United States, third among the top 10 countries for number of adults with the disease, IDF calculates $348 billion of healthcare spending for diabetes in 20171 (Figure 1). The American Diabetes Association (ADA) estimate is similar: $327 billion in U.S. spending in 2017, $237 billion for direct medical costs and $90 billion in reduced productivity, a 26% increase from 2012.2
As the need for diabetes-related health care continues to escalate, so does the economic impact on individuals and the medical practices that serve them. People with diabetes, on average, have medical expenditures 2.3 times higher than what expenditures would be in the absence of diabetes.2 For retina practices, the economic impact is felt primarily through increasing patient volume and delivery of anti-VEGF injections. Some reach the point where hiring additional physicians or staff is necessary. Others improve practice flow by adding injection rooms or altering the path patients take through the practice to shorten their walk between rooms. Another option, supported by recent clinical trials,3,4 is to consider anti-VEGF treatment for moderately severe to severe nonproliferative diabetic retinopathy whether or not it’s accompanied by diabetic macular edema (DME) to prevent disease progression and lessen the need for future treatment. Often, treatment with a goal to regress retinopathy requires a lower treatment volume than treatment to improve DME. Retina specialists also report the following steps that can be taken to help maintain practice efficiency and to help patients afford their treatments.
- Use pre-filled syringes. A ranibizumab (Lucentis, Genentech) pre-filled syringe for use in treating diabetic retinopathy has been approved by the FDA, and an aflibercept (Eylea, Regeneron) pre-filled syringe is expected to receive approval by the end of this year. Arshad Khanani MD, MA, managing partner and director of clinical research at Sierra Eye Associates in Reno, NV, has been using the Lucentis pre-filled syringe. “I draw my own drug from the vial, and the pre-filled syringe definitely decreases my prep time and is very efficient,” he says. “In addition, there are less steps involved in injection prep, which can likely lower the risk of endophthalmitis.5 I love it. I save 30 or so seconds each time, which adds up [when administering] 30 to 40 injections in a day.”
Surgeons at two centers who evaluated the Lucentis pre-filled syringe found it saved them 17 seconds and 29 seconds per procedure, respectively, a 27% to 39% reduction in syringe preparation time compared with when they used the standard vial method.6 Another group assessed the usability of the Lucentis pre-filled syringe by retina specialists and ophthalmic medical personnel in simulated and actual-use settings. Participants completed all injection-related tasks without any critical errors and reported the syringe was easy or very easy to use.7
- Schedule injection-only visits or refer routine visits. For patients already on an anti-VEGF treatment plan, Dr. Khanani doesn’t dilate at every visit. Instead, he says, “If the patient is stable and has no hemorrhage, he or she comes in for OCT, visual acuity, and IOP checks, then receives an injection and goes home. These visits are faster because patients don’t have to wait to be dilated.”
Nancy Holekamp, MD, director of retina services for Pepose Vision Institute in Chesterfield, MO, is employing a different strategy: “We’re seeing only patients who need treatment and referring those who don’t to their primary eyecare provider for their routine diabetic visits.” - Think “outside the box.” David Eichenbaum, MD, and his partners at Retina Vitreous Associates of Florida, expanded their efficiency efforts to outside the building. “At some of our free-standing locations with small parking lots, we use valet services so patients aren’t spending time driving around looking for a space or potentially giving up and leaving.”
- Enroll patients in assistance programs and establish a paperwork routine. Genentech and Regeneron offer comprehensive programs to help ensure patients with commercial insurance have access to their medications for on-label indications. Eylea4U and Lucentis Access Solutions offer co-pay assistance regardless of income (covering up to $10,000 or $15,000 per year and reimbursing practices directly), other forms of payment assistance based on financial need, and tools and resources to support patients and help them manage their disease. Once the practice completes an enrollment form and sends an Explanation of Benefits, both companies’ programs evaluate a patient’s benefits, determine eligibility for assistance, and communicate with the patient and the practice accordingly. Both companies also provide online portals practices can use to enroll and track their patients through the programs.
“We put every single patient into an assistance program,” explains Dr. Holekamp. “Once you have a process set up in your practice, you can just plug patients into it. When you’re doing it for everybody, the staff get very good at it. And it’s best to use the online portals. With login credentials, you can check it from any computer in the practice.”
“These programs are really good,” Dr. Khanani says. “There is some work to be done by the practice, but that’s a small price on our end to make sure patients get the right treatment and don’t lose vision.” ■
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation, 2017.
- American Diabetes Association. The cost of diabetes. Available at: http://www.diabetes.org/advocacy/news-events/cost-of-diabetes.html ; last accessed March 5, 2019.
- Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2018;136(10):1138-1148.
- Regeneron Pharmaceuticals, Inc. One-year results from positive phase 3 Eylea trial in diabetic retinopathy presented at angiogenesis symposium]. Available at: https://investor.regeneron.com/news-releases/news-release-details/one-year-results-positive-phase-3-eylea-trial-diabetic ; last accessed March 5, 2019.
- Storey PP, Tauqeer Z, Yonekawa Y, et al.; Post-Injection Endophthalmitis (PIE) Study Group. The impact of prefilled syringes on endophthalmitis following intravitreal injection of ranibizumab. Am J Ophthalmol. 2019 Mar;199:200-208.
- Souied E, Nghiem-Buffet S, Leteneux C, et al. Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures. Eur J Ophthalmol. 2015;25(6):529-534.
- Antoszyk AN, Baker C, Calzada J, et al. Usability of the ranibizumab 0.5 mg prefilled syringe: human factors studies to evaluate critical task completion by healthcare professionals. PDA J Pharm Sci Technol. 2018;72(4):411-419.








