Current evidence-based management of diabetic eye disease centers around the proven efficacy of anti-VEGF agents for the treatment of diabetic macular edema (DME). The RISE/RIDE and VIVID/VISTA phase 3 clinical trials demonstrated the safety, effectiveness, and superiority over laser treatment of ranibizumab (Lucentis 0.3 mg, Genentech)1 and aflibercept (Eylea 2 mg, Regeneron).2 Three-year data from the trials showed that with frequent injections (Lucentis monthly; Eylea every 8 weeks after 5 monthly injections), the favorable anatomic and visual acuity (VA) results achieved can be maintained. While the most robust results have been achieved with the consistent dosing that was used in the clinical trials, as-needed treatment protocols utilizing Lucentis, e.g. those followed in the RESTORE, Diabetic Retinopathy Clinical Research Network (DRCR.net ) Protocol I, and RISE/RIDE extension studies, have also been shown to effectively reduce retinal thickness and improve VA in eyes with DME.3-5 Subsequent to the pivotal anti-VEGF trials, The DRCR.net Protocol T study, a direct comparison of Lucentis, Eylea, and off-label bevacizumab (Avastin, Genentech), showed all three agents to be safe and effective treatments for DME.6 In a pre-specified subgroup analysis from Protocol T, Eylea outperformed the other drugs at 1 year for eyes with baseline visual acuity of 20/50 or worse. At 2 years, the advantage of Eylea in that subgroup was no longer statistically significant.
Intravitreal sustained-release steroid implants are another option for achieving anatomic improvements and vision gains in eyes with DME, as evidenced by the clinical trials that led to FDA approval of dexamethasone 0.7 mg (Ozurdex, Allergan) and fluocinolone acetonide 0.19 mg (Iluvien, Alimera Sciences). Although the percentages of patients who achieved a ≥15-letter VA improvement in the 3-year MEAD trial of Ozurdex and the 3-year FAME trial of Iluvien weren’t as high as were seen in the anti-VEGF trials, they were achieved with fewer treatments: an average of one implant over 3 years in FAME7 and 4.1 implants over 3 years in MEAD.8 A subgroup analysis from the FAME trial yielded further guidance on the use of Iluvien. Vision outcomes were best in patients with DME duration of 3 or more years at baseline (34% gained 15 or more letters of vision).9
Focal laser remains an option for reducing the risk of vision loss in some cases of DME.10 However, given its potential to cause collateral damage to vision and the availability of alternative therapies more likely to improve vision, it’s typically applied only outside the center of the macula. In the Protocol I study, in eyes with DME involving the central macula and vision impairment, focal or grid laser treatment delivered at the start of anti-VEGF therapy produced results no better than deferring laser treatment for 24 or more weeks.4 Focal laser proved useful in the READ-2 study in which it reduced the need for more frequent anti-VEGF injections for controlling DME.11
For high-risk proliferative diabetic retinopathy (PDR), as established by the Diabetic Retinopathy Study, panretinal photocoagulation (PRP) reduces the risk of severe vision loss.12 Despite its potential side effects, PRP may be advantageous for patients with PDR expected to be noncompliant with recommended follow-up. In a study of patients with PDR who were treated with either anti-VEGF injections or PRP prior to being lost to follow-up for more than 6 months, the PRP group had better anatomic and functional outcomes when they returned for care.13 With laser’s continuing role in diabetic eye care have come technological improvements designed to maximize effectiveness and efficiency and minimize side effects. Pattern-scanning lasers enable more precise, predictable, and efficient delivery of PRP. For DME, advances include computer-navigated systems to improve delivery accuracy and shorter-duration pulses that have shown the capability to reduce macular edema with no discernible tissue damage.14
Complexity of Diabetic Eye Disease Requires a Multi-pronged Approach
In clinical practice, the complexity of diabetic eye disease often requires more than anti-VEGF monotherapy for controlling progression. In the Protocol I study, approximately 50% of patients responded well to anti-VEGF treatment and approximately 50% of patients did not,4 and the number of injections was not responsible for the different responses.
At 2 years in the protocol T study, 41% of patients receiving Eylea and 52% of patients receiving Lucentis had received at least one laser treatment per the protocol.15 The fact that not all patients with DME respond satisfactorily to anti-VEGF treatment serves as the rationale for switching or combining treatments, often an anti-VEGF agent followed by or in conjunction with a sustained-release steroid implant. In PDR, both anti-VEGF injections and PRP are often used together to control disease progression.
An analysis of data from the Protocol I study, referred to as the EARLY analysis, indicated that how a patient’s DME responds to three monthly anti-VEGF injections predicts the long-term response,16 giving physicians an opportunity to change treatment strategy as soon as 12 weeks for those patients who don’t achieve a positive outcome. The DRCR Protocol U study aimed to determine whether adding Ozurdex (every 3 months rather than per label) to anti-VEGF therapy could lead to improved vision outcomes at week 24 for patients with persistent DME. At 24 weeks, no overall difference in visual acuity was found between the steroid plus anti-VEGF group and the anti-VEGF alone group.17 However, the mean decrease in central subfield thickness in the combination group was significantly greater. In addition, DME was completely resolved in nearly half of the eyes in the combination group, compared with 31% of the anti-VEGF alone group.
According to Michael A. Singer, MD, director of clinical research at Medical Center Ophthalmology Associates in Texas, the VA and retinal thickness findings in Protocol U and other studies point to an important, still-open question: What is the value of a dry retina?
“My analyses of data from the FAME and other trials indicate that patients with dry retinas have better visual function questionnaire scores,” Dr. Singer says. “Fully resolved DME may be under-appreciated at the moment. If a dry, flat retina is what dictates good vision, which is what patients want, and what payers judge as well, we may need to adjust our treatment goals, and combination therapy with steroids may play a larger role.”
The Latest Data Prompt Re-evaluation of When to Intervene
Best practices in diabetic eye care involve not only which treatments to use, but also at what point initial treatment should begin. Currently, the accepted approach is to observe until DME is affecting vision or PDR or high-risk PDR develops. But, as more trial data has been obtained, a new question has arisen: Should treatment be initiated sooner — i.e., at a nonproliferative disease stage — when anti-VEGF therapy could reverse DR or prevent its progression? Lucentis was recently approved by the FDA for treating all forms of DR, with or without DME, and a similar approval is expected for Eylea.
With respect to DME, the pivotal trials of Lucentis and Eylea1,2 indicated that earlier treatment produces the best vision outcomes. The patients who received sham or laser treatment, when crossed over to anti-VEGF treatment after 24 months, had a poorer VA response and never caught up to the response in the patients who were anti-VEGF-treated from the outset.
“This is a fairly consistent finding throughout all of our studies of anti-VEGF agents in DME,” notes Nancy Holekamp, MD, director of retina services for Pepose Vision Institute in Chesterfield, MO. “The earlier you treat, the better they do.” Dr. Holekamp cites the Protocol U study as additional support for early treatment of DME. By the time a steroid implant was added to anti-VEGF therapy, patients had already received as many as 12 injections while edema persisted. “Although the anatomy improved with the change in treatment approach, patients on average didn’t see any better because ineffective treatment is tantamount to no treatment. If treatment is delayed long enough, the retina may suffer irreversible damage, even if the anatomy improves.”
Evidence of the ability of anti-VEGF agents to regress DR has been seen in several trials and analyses, including the following:
- In a secondary analysis from the VIVID DME trial, 32.6% of patients treated with Eylea every 8 weeks had a two-step or more improvement in Diabetic Retinopathy Severity Scale (DRSS) score at week 100 compared with 8.2% of laser-treated patients.18 In a secondary analysis from the VISTA DME trial, 37.1% of patients treated with Eylea every 8 weeks had a two-step or more improvement in DRSS score at week 100 compared with 15.6% of laser-treated patients. In both trials, fewer Eylea-treated patients than laser-treated patients progressed to PDR.18
- A post hoc analysis from the RISE/RIDE DME trials examined DR outcomes by baseline severity level. At month 24, treatment with Lucentis resulted in DRSS score improvement in all severity subsets that were examined. The highest rates of improvement of two or more DRSS steps were seen among patients with baseline DR levels 47/53: moderately severe to severe nonproliferative DR (NPDR) (Lucentis 0.3 mg, 78.4%; Lucentis 0.5 mg, 81.1%; and sham, 11.6%).19 Also among patients with 47/53 baseline DR severity, Lucentis treatment reduced the probability of a new proliferative event at month 36 by 3 times compared with sham.
- The Protocol S trial conducted by DRCR compared Lucentis to PRP in patients with PDR with and without DME. At 2 years and 5 years, VA in the two treatment groups was similar.20,21 At the 2-year mark, among patients who had DME at baseline and, therefore, received at least one mandatory Lucentis injection, 58.5% achieved a two-step or more improvement in DRSS score and 31.7% achieved a three-step or more improvement. Also at 2 years, among patients who did not have DME at baseline, but were randomized to receive Lucentis for PDR, 37.8% achieved a two-step or more improvement in DRSS score and 28.4% achieved a three-step or more improvement. At 5 years, Lucentis was associated with the advantages of less visual field loss and lower rates of development of vision-impairing DME. The 5-year data analysis of retinopathy improvement by baseline DME status has not yet been published. However, overall, 46% of patients treated with Lucentis achieved a two-step or more improvement in DRSS score based on fundus photographs (Figure 1).
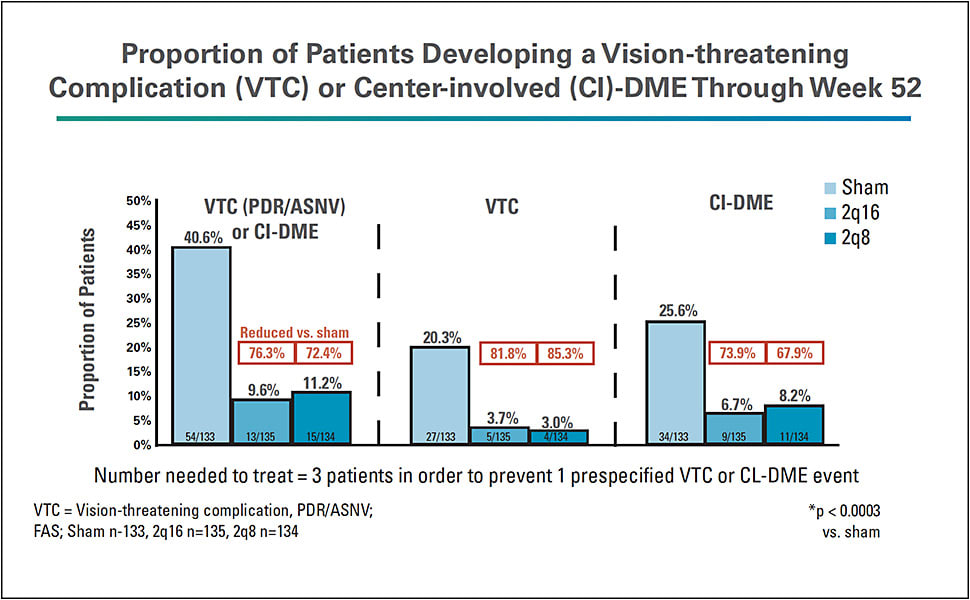
- The PANORAMA trial compared Eylea to sham in patients with moderately severe and severe NPDR without DME. Both treatment groups received initial monthly dosing. Subsequently, one group received Eylea every 8 weeks and the other group received Eylea every 16 weeks. At the 52-week endpoint, 80% of patients who received Eylea every 8 weeks and 65% of patients who received Eylea every 16 weeks experienced a two-step or more improvement in DRSS score compared with 15% of patients who received sham (Figure 2).22 In secondary endpoint analyses, 41% of the sham group developed a vision-threatening complication (PDR or anterior segment neovascularization) or center-involved DME compared with 11% of the group that received Eylea every 8 weeks and 10% of the group that received Eylea every 16 weeks (Figure 3). Among untreated patients with severe NPDR, 53% developed a vision-threatening complication at 52 weeks. Eylea treatment reduced the risk of these events by approximately 74% compared with sham injection.


David Eichenbaum, MD, a partner with Retina Vitreous Associates of Florida, was part of the writing team for the RISE/RIDE DR subanalysis paper. “The data are compelling,” he says. “And we’re seeing repetition of the science across studies, which is good. It seems that diabetic retinopathy is most sensitive to treatment with anti-VEGF agents at the moderately severe and severe nonproliferative stages. The 47/53 DRSS levels are where anti-VEGF treatment produces the most profound amount of disease regression. In fact, a significant number of patients regress three steps when treated at that point, which is highly clinically meaningful regression. PANORAMA confirmed this for moderately severe and severe NPDR without DME as well. Protocol S also showed significant regression in the PDR population, but to a less substantial degree than the 47/53 severe NPDR population. Furthermore, while RISE/RIDE and VIVID/VISTA supported a relatively high protocol treatment burden because the target was DME, Protocol S and PANORAMA showed regression in mostly non-DME patients with less frequent injections.”
Implementing an Evolving Treatment Paradigm
As retina specialists consider the evolving treatment paradigm, intervening when NPDR is moderately severe or severe, they’re also considering the related challenges, particularly patients’ willingness to undergo treatment when they’re asymptomatic. Making earlier intervention a reality may also be challenging given that utilization of anti-VEGF injections for diabetic eye disease in clinical practice is already lower than in clinical trials, a finding drawn from many analyses of health and insurer databases.23-26
“Analyzing the ‘big data’ shows us how clinical trial data is being incorporated in real life, how we’re actually treating patients,” Dr. Singer says.
Another consideration is the tendency for diabetic patients to be lost to follow-up. For example, a recent retrospective study involving 2,595 NPDR patients with DME treated with at least one anti-VEGF injection at a large retina practice revealed approximately 25% had no follow-up office visit for at least 1 year after an injection.27
“We know that patients with moderately severe or severe NPDR are at significant risk for progression to proliferative retinopathy or DME within a year,” says Dr. Singer. “But we also know loss to follow-up is a real concern for diabetic patients. They deal with significantly more doctor visits than other types of patients, which makes compliance difficult. Patients treated with anti-VEGF injections need to be followed, and, right now, we don’t know how long we need to treat them in order to permanently regress the retinopathy. Also, it would be of great value to know to what degree resolution of retinopathy improves quality of life. That would be a big motivator.”
Drs. Singer and Eichenbaum both say they’re likely to intervene with injections earlier than they would have in the past when one eye is significantly more affected and the second eye has asymptomatic DR. Patients in that circumstance are more open to injections in their better eye because they’ve experienced what has happened due to diabetic effects in the first eye.
“What changed for me in the past year or so is that I’m offering treatment to patients with moderately severe or severe nonproliferative diabetic retinopathy, especially if they’ve lost some vision or are doing significant battle with a diabetes-related problem in their other eye,” says Dr. Eichenbaum. “I tell them that based on their worse eye, we know where their good eye is headed, and, by treating now, we can probably prevent that from happening — at least for a while.”
Overall, notes Dr. Singer, “I definitely start the conversation about injections much sooner than I had in the past.”
Arshad Khanani MD, MA, director of clinical research with Sierra Eye Associates in Nevada, agrees. “I have the conversation up front now with patients who have moderately severe or severe NPDR,” he says. “We know about half of the patients with severe NPDR will go on to proliferative disease or DME. In my practice, the majority of these patients don’t want treatment at this stage due to the treatment burden, so we continue to follow them closely. For those who have even a small amount of DME, it’s better to treat them now versus later because we know as the retinopathy progresses, the edema will get worse, and we can prevent that progression.”
Review of Guidelines for Administration of Intravitreal Injections
During the Hawaiian Eye & Retina 2019 meeting, Sophie Bakri, MD, presented, “Review of Guidelines for Administration of Intravitreal Injections.” The guidelines are based on deliberations by an expert panel of ophthalmologists conducted after a literature review.1
Dr. Bakri’s presentation included guidelines pertaining to endophthalmitis prevention, some of which have evolved over time:
- Gloves, sterile or nonsterile, aren’t required. Neither OSHA nor CDC require gloves for injections, although patients may expect healthcare providers to wear gloves. Handwashing is, of course, essential before and after each patient.
- Pupil dilation is optional but not necessary. Some panel members believe the documented presence of formed vision post-injection is sufficient. Others maintain that pupil dilation and post-injection examination of the posterior segment is important.
- Draping the patient, which may increase patient discomfort, is an additional cost that has not been shown to reduce infection risk. Thus, it is discouraged but optional.
- Aqueous chlorhexidine (0.05%, not 0.5%) can be used as an alternative to povidone-iodine in patients who report an iodine allergy. Allergy to povidone-iodine is extremely rare, and many patients may not know that seafood allergies aren’t related to iodine. A skin patch test can be performed in cases of undocumented allergy claims, but the panel does not support routine testing. When anesthetic gel is used, chlorhexidine and povidone-iodine should be applied to the injection site before and after gel application.
- Patients and physicians should minimize talking (or the physician can wear a mask) as the injection is administered to prevent contamination by the common respiratory flora that have been cultured in patients diagnosed with endophthalmitis following intravitreal injections.
- There is insufficient evidence to support routine use of topical antibiotics for endophthalmitis prevention before, during, or after an intravitreal injection.
Reference
- Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34 Suppl 12:S1-S18.
A Change in Conventional Wisdom
Dr. Holekamp summarizes where conventional thinking and the future of diabetic eye care converge, “If DME is present, that’s our priority for treatment. And while we treat the DME with anti-VEGF injections, the retinopathy will improve. In the absence of DME, we should be thinking about and targeting moderately severe and severe NPDR, because we can prevent progression.”
Retina specialists look forward to results from ongoing studies examining early intervention. The DRCR Protocol W trial will shed more light on the efficacy of Eylea for prevention of PDR and center-involving DME, including comparison of long-term visual outcomes in eyes that receive early anti-VEGF therapy versus eyes initially observed.28 DRCR.net Protocol V will provide guidance on treatment of center-involving DME in eyes with good visual acuity.29 ■
References
- Brown DM, Nguyen QD, Marcus DM, et al., on behalf of the RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials. Ophthalmology. 2013;120:2013-2022.
- Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376-2385.
- Schmidt-Erfurth U, Lang GE, Holz FG, et al., on behalf of the RESTORE Extension Study Group. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121:1045-1053.
- Elman MJ, Qin H, Aiello LP, et al., The Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt vs deferred laser treatment: 3-year randomized trial results. Ophthalmology. 2012;119:2312-2318.
- Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy; long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122:2504-2513.
- Wells JA, Glassman AR, Ayala AR, et al., The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
- Campochiaro PA, Brown DM, Pearson A, et al. FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125-2132.
- Boyer DS, Yoon YH, Belfort R Jr, et al., Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
- Cunha-Vaz J, Ashton P, Iezzi R, et al. FAME Study Group. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121(10):1892-1903.
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987;94(7):761-774.
- Do DV, Nguyen QD, Khwaja AA, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013 Feb;131(2):139-145.
- The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of diabetic retinopathy study (DRS) findings. DRS Report 8. Ophthalmology. 1981;88(7):583-600.
- Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2018 Aug 2. pii: S0161-6420(18)31079-0. doi: 10.1016/j.ophtha.2018.07.027 [Epub ahead of print].
- Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89(1):74-80.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of Protocol I study data. Retina. 2019;39(1):88-97.
- Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29-38.
- Mitchell P, McAllister I, Larsen M, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmology Retina. 2018;2:988-996.
- Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmology Retina. 2018;2:997-1009.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy - a randomized clinical trial. JAMA. 2015;314(20):2137-2146.
- Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2018;136(10):1138-1148.
- Regeneron. One-year results from positive phase 3 Eylea trial in diabetic retinopathy presented at angiogenesis symposium. Available online: https://investor.regeneron.com/news-releases/news-release-details/one-year-results-positive-phase-3-eylea-trial-diabetic ; last accessed Mar. 5, 2019.
- Holekamp N, Campbell J, Cole A, et al. Real-world vision outcomes in DME treated with anti‐VEGF injections - an analysis of EMR data from a large health system. Paper presented at: American Society of Retina Specialists annual meeting; August 2014; San Diego, CA.
- Fong D, Luong T, Jimenez J, Contreras R, Campbell J, Patel V. Visual acuity outcomes in patients treated with anti-VEGF therapy for diabetic macular edema in a US integrated healthcare system. Paper presented at: Macula Society annual meeting; February 2015; Scottsdale, AZ.
- Kiss S, Liu Y, Brown J, Holekamp N, Almony, A, Campbell J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611-1621.
- Holekamp N, Dugel P, Yep T, et al. Utilization of anti-VEGFs for diabetic macular edema in US clinical practice - an analysis of the Vestrum Health database. Paper presented at: Retina Society annual meeting; October 2015; Paris, France.
- Gao X, Obeid A, Aderman CM, et al. Loss to follow-up after intravitreal anti-vascular endothelial growth factor injections in patients with diabetic macular edema. Presented at: American Society of Retina Specialists; August 2017; Boston, MA.
- Diabetic Retinopathy Clinical Research Network. Protocol W: Intravitreous anti-VEGF treatment for prevention of vision threatening diabetic retinopathy in eyes at high risk. Available at: https://public.jaeb.org/drcrnet/stdy/340 ; last accessed Mar. 5, 2019.
- Diabetic Retinopathy Clinical Research Network. Protocol V: Treatment for central-involved diabetic macular edema in eyes with very good visual acuity. Available at: https://public.jaeb.org/drcrnet/stdy/233 ; last accessed Mar. 5, 2019.








