With an agent in the anti-VEGF class now FDA-approved for use in all forms of diabetic retinopathy (DR), retina specialists can consider implementing their preferred first-line treatment modality earlier in the disease course. The data imply earlier is better for safeguarding vision, and ocular imaging is playing an increasingly important role. Today’s diagnostic tools are providing wider, deeper, and clearer views of the retina, thus rendering a more accurate assessment of the ocular effects of diabetes. Imaging technologies are revealing new biomarkers and making it easier to obtain images of relevant pathology. Not only do these developments aid earlier detection of diabetic eye disease and improve prognostic accuracy, they may, someday, change risk stratification and guide more individualized treatment choices.
Widefield Fundus Photography and Angiography
The latest color fundus cameras have widened the view of the retina considerably. With ETDRS seven-field fundus photography, seven individually captured 30° images can be montaged to yield an approximately 75° field of view. Widefield and ultra-widefield systems can capture up to 200° with a single image, and even wider fields of view with montaging. Views up to 200° are also obtainable with widefield and ultra-widefield fluorescein angiography (FA) and indocyanine green angiography.
Widefield imaging has helped to confirm the extent to which diabetic eye disease can manifest outside the posterior pole and has also improved diagnosis (Figures 1 and 2). For example, in an analysis comparing ETDRS seven-field imaging with ultra-widefield FA in patients with DR, the latter showed 1.9 times more neovascularization, 3.9 times more retinal nonperfusion, and 3.8 times more panretinal photocoagulation (PRP).1 In 10% of eyes, ultra-widefield FA detected pathology, including retinal nonperfusion and neovascularization, that wasn’t evident based on the standard imaging.
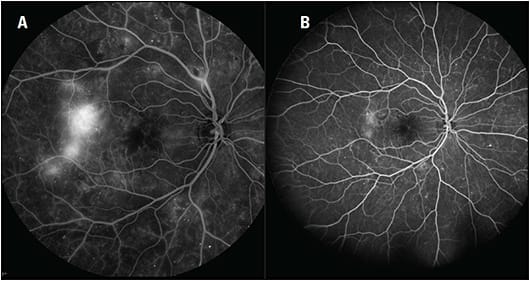
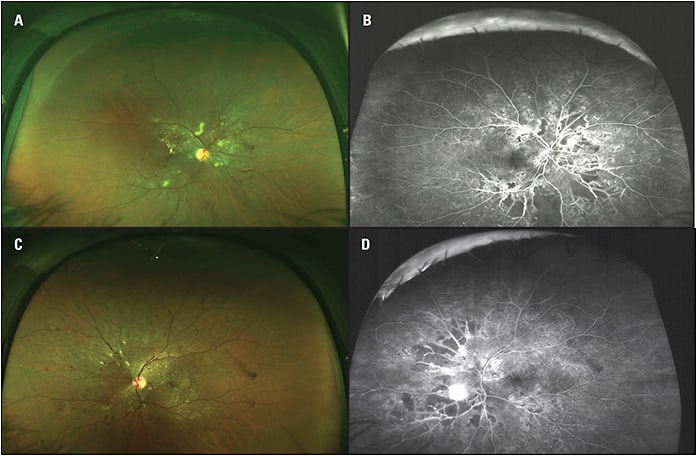
Arshad Khanani, MD, MA, managing partner and director of clinical research at Sierra Eye Associates in Reno, NV, has been using widefield imaging routinely for 5 years and considers it crucial for diabetic eye care.
“Without widefield, it’s possible to miss pathology,” he says. “With widefield, I get a much better assessment of the status of the retinal vasculature.”
Furthermore, explains Nancy Holekamp, MD, director of retina services for Pepose Vision Institute in Chesterfield, MO, “We have come to appreciate that the peripheral retinal exam is critical to predicting which patients are likely to experience progression of diabetic retinopathy.”
As documented by Silva and colleagues, the presence and extent of predominantly peripheral lesions (PPLs) in diabetic eyes, i.e., lesions with a greater extent outside versus inside standard ETDRS fields, are associated with increased risk of DR progression.2 His study group identified PPLs in baseline ETDRS seven-field fundus images and ultra-widefield fundus images from patients who were previously enrolled in a comparative instrument validation study.
Four years later, they obtained follow-up ETDRS images. In eyes without proliferative diabetic retinopathy (PDR) at baseline, 51% had at least one field with PPLs, and 39% had DR progression at 4 years. Compared with eyes without PPLs, eyes with PPLs had a 3.2-fold increased risk of DR progression of two steps or more and a 4.7-fold increased risk of progression to PDR. As the extent of fields containing PPLs increased, so did the risk of two-step or more progression and progression to PDR.
In another study, researchers used ultra-widefield FA to identify PPLs and determine the extent of retinal nonperfusion area (NPA) and calculate nonperfusion index (NPI, nonperfused/total gradable area) in diabetic patients with and without DR. Evaluating relationships between the identified biomarkers and with DR severity, they found PPLs to be associated with increased NPA and NPI, including when adjustments were made for DR severity and duration of diabetes.3 In eyes that did not have PDR, increasing NPA and NPI values were associated with worsening DR.3 The researchers concluded, “Given that the presence and extent of PPLs have been associated with increased risks of DR progression, the clinical identification of PPLs may reflect closely the extent of nonperfusion and ischemia, thus accounting for the increased risk of progression.”
Ischemia in the retinal periphery has also been linked to diabetic macular edema (DME).1 For example, in a group of treatment-naïve patients with diabetic retinopathy who were imaged with ultra-widefield FA and determined to have DME or not based on OCT and clinical exam, eyes with peripheral ischemia were 3.75 times more likely to have DME.4
The findings surrounding widefield imaging, earlier detection of diabetic eye disease, and the predictive value of the retinal periphery have become a factor in clinical decision-making. Says Dr. Khanani, “With widefield imaging, I diagnose both severe nonproliferative and proliferative DR earlier than on exam alone. Diagnosing sooner means I can intervene sooner with PRP or anti-VEGF to prevent disease progression and vision loss.”
For peripheral neovascularization discovered with widefield imaging, David Eichenbaum, MD, a partner with Retina Vitreous Associates of Florida, always recommends treatment.
“While peripheral neovascularization wasn’t evaluated in the landmark studies of diabetic eye disease, it’s still a sign of significant disease,” he says. “It makes sense to extrapolate the data we have from more traditional imaging settings to what we’re seeing with widefield imaging.”
Based on the current evidence, Dr. Eichenbaum says any peripheral pathology makes him apt to consider recommending treatment for a diabetic patient.
“The ANDROID study,5 albeit a small study, showed that serial anti-VEGF injections can improve peripheral nonperfusion in proliferative retinopathy and/or DME,” he notes. Dr. Eichenbaum looks forward to the results from the DRCR Protocol AA study, which are expected next year. Protocol AA is evaluating peripheral lesions and the risk of DR worsening over time.6 “I think it’s going to be an extremely helpful result and will solidify what we’re seeing in smaller and retrospective studies,” he says. “We’re developing prospective, level-one evidence related to the peripheral retina, and I believe, in general, we’re going to want to treat patients with peripheral pathology more aggressively earlier.”
Noting the new indication for ranibizumab (Lucentis, Genentech), DR with or without DME, and the same expected for aflibercept (Eylea, Regeneron) soon, Michael A. Singer, MD, director of clinical research at Medical Center Ophthalmology Associates in Texas, says that now, when doctors see disease in the periphery, they can do something about it.
“At the least, we know to follow these patients more closely,” he says. “If we’re looking for peripheral disease earlier, patients are much less likely to ‘fall through the cracks,’ and then it’s up to individual practitioners to determine their threshold for recommending treatment.”
OCT Angiography
OCT angiography (OCTA) is one of the newest imaging technologies available to retina specialists. Unlike FA, it doesn’t rely on the use of intravenous dye. As such, it’s a safer and faster diagnostic test. Using motion contrast to detect blood flow, it provides detailed three-dimensional visualization of the retinal and choroidal vasculature. It has demonstrated the ability to identify features salient in DR, such as neovascularization, microaneurysms, intraretinal microvascular abnormalities (IRMA), and nonperfusion.7
The abilities of OCTA to capture high-resolution views of the retinal capillaries and to separately image the superficial capillary plexus and the deep capillary plexus8 may offer an advantage over FA in evaluating DR. The fact that OCTA doesn’t rely on dye or show leakage from vessels furthers this advantage. Capillary details, including areas of dropout, aren’t obscured by leakage as they may be on FA.9 OCTA is also a more proficient detector of early retinal neovascularization.9 Areas of focal leakage that appear to be microaneurysms on FA can be identified by OCTA as neovascularization.9 Furthermore, algorithms developed for OCTA automatically quantify parameters related to DR, such as vessel density, size of the foveal avascular zone (FAZ), and area of nonperfusion. Notes Dr. Singer, “OCTA provides a better understanding of diabetic retinopathy at the intraretinal and subretinal level, particularly perfusion status.”
Many studies are exploring whether OCTA-measured retinal nonperfusion is an easily obtainable, objective proxy for the severity of DR as determined by conventional means and could, therefore, serve as a biomarker for following the progression of the disease. One such study compared OCTA perfusion index (percent coverage of functional retinal capillaries in the traditional ETDRS zones) in diabetic patients who had varying severities of DR. Perfusion index was significantly lower in all nonfoveal ETDRS zones in eyes with moderate or severe nonproliferative diabetic retinopathy (NPDR) or PDR compared with perfusion index in eyes with no or mild NPDR, suggesting a correlation between capillary perfusion and severity of diabetic retinopathy.10 In another study that produced similar results, decreases in perfusion index as NPDR progressed were more pronounced and occurred sooner in the deep capillary plexus compared with the superficial plexus.11
Other studies have indicated OCTA may be useful for detection of DR prior to the appearance of clinical signs. Eyes of diabetic patients without apparent retinopathy have been shown to exhibit on OCTA reduced average vessel density in the superficial and/or deep capillary plexus and/or choriocapillaris12,13 and a larger foveal avascular zone (FAZ)14,15 when compared with normal eyes (Figure 3). In a study involving subjects younger than 40 with type 1 diabetes and DR requiring PRP, but not macular edema, the degree of capillary loss in the deep plexus was associated with decreased visual acuity.16
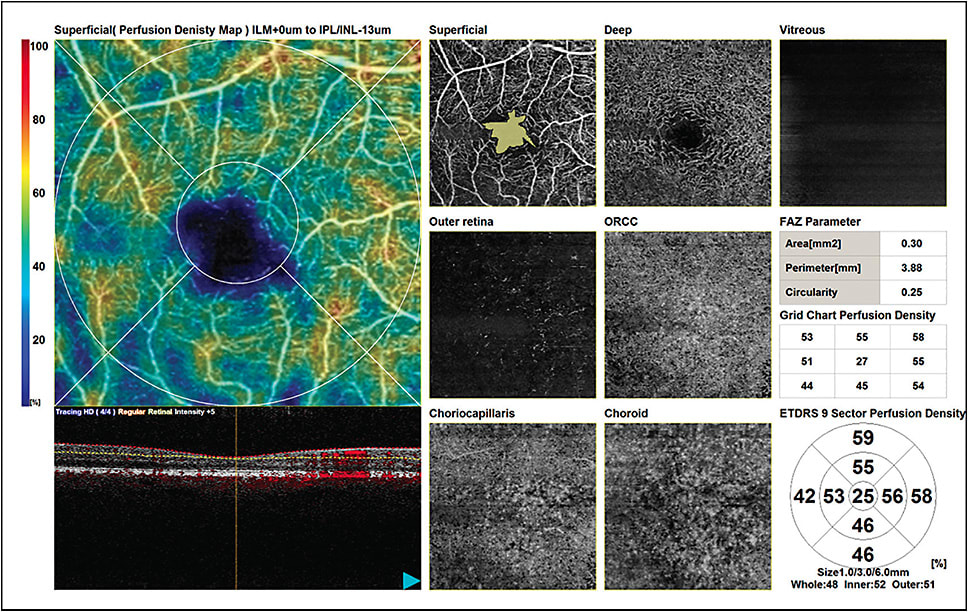
With further validation of biomarkers and continued improvement in the technology, OCTA is expected to play an increasing role in clinical decision-making. Advances, such as measurement of blood flow speed rather than the current flow/no flow determination, are emerging. Now and in the future, a chief benefit of OCTA is its noninvasiveness, which allows frequent patient monitoring unhindered by safety or time concerns. Dr. Eichenbaum acknowledged some strengths and shortcomings of the OCTA devices available to clinical practices today.
“They very elegantly capture nonperfusion in the posterior pole, probably better than dye-based angiography, providing the ability to follow it quantitatively over time,” says Dr. Eichenbaum. “We can see if those areas enlarge or regress with various treatment modalities. There’s improvement to be made in the consistency of reading the vessels in the presence of significant macular edema due to segmentation problems and projection artifacts when the edema is more severe. And currently the area that can be imaged with ideal resolution is relatively small. As faster scanners are incorporated and more powerful processors that can quickly ‘crunch’ all the data are introduced, we’ll get wider OCTA images.”
Expanding the Uses of OCT
While OCT is the gold standard in diagnosing and monitoring DME and assessing the need for surgical intervention in advanced DR, the search is on for different retinal disease characteristics, both neurovascular and morphological, that may serve as biomarkers for earlier detection of diabetes-related damage and, perhaps, correlate more reliably with visual acuity. One focus is OCT’s ability to measure thickness changes in the neuroretina, which a body of research suggests may precede vascular changes.
Studies have shown that eyes of patients with diabetes and no or minimal signs of DR have decreased thickness of the central fovea,17 central fundus,17 ganglion cell/inner plexiform layer,18 inner nuclear layer,18 pericentral area of the central macula,18 ganglion cell layer,19 and retinal nerve fiber layer (RNFL),17,19 compared with healthy, matched control eyes. Sohn and colleagues reported significant, progressive loss of RNFL and the ganglion cell/inner plexiform layer over a 4-year period in diabetic patients exhibiting no or minimal clinical signs of DR.20 The loss was independent of glycated hemoglobin, age, and sex. The same group also documented significantly thinner RNFL in donor eyes of diabetic patients with no or minimal DR compared with healthy donor eyes in which retinal capillary density (determined by ImageJ processing) did not differ.20
Vujosevic and colleagues compared OCT metrics with OCTA metrics in patients with diabetes but no signs of DR and healthy control subjects. They found several neural and microvascular modifications to be present prior to clinical signs of DR and also that perifoveal capillary loss in the superficial capillary plexus and inner retinal layer thickness were the most highly correlated of the metrics.21 Also in patients with diabetes but no or minimal DR, ganglion cell layer thickness has been shown to be an independent predictor of decreased visual function.22
Another OCT finding of interest in diabetic patients is disorganization of the retinal inner layers, referred to as DRIL. DRIL is a potential biomarker for visual acuity in eyes with center-involved DME and a potential predictor of poor response to treatment.23-26 In addition to visual acuity in eyes with DME, DRIL has been associated with disruption of the outer retina and increasing DR severity.27
According to Dr. Khanani, “We don’t have consensus yet on the exact impact of DRIL, but, anecdotally, it’s fairly clear that patients with DRIL and significant edema and damaged photoreceptor inner/outer segments tend not to do very well.”
New thoughts about OCT findings related to the choroid are also of interest, Dr. Eichenbaum says. With the use of OCT enhanced-depth imaging strategies, subfoveal choroidal thickness in diabetic patients with and without DR has been shown to be reduced and potentially related to retinopathy severity compared with healthy controls.28-30 “These findings are interesting and likely speak to the oxygen diffusion in diabetes,” he says. “It seems when there is ischemia in the retina, similar changes are seen in the choroid. So, the question is whether modulating choroidal thickness somehow, perhaps using the suprachoroidal space for drug delivery, could affect the advancement of diabetic retinopathy. It’s still very exploratory, but the choroid could turn out to be a therapeutic target.”
The More Information, the Better
“My mainstays for detecting early diabetic retinopathy and DME are still the 90-diopter lens, fluorescein angiography, OCT B-scans, and color fundus photos,” Dr. Eichenbaum continues. “But, when we have validated treatment guidance surrounding the newer findings from our extremely high-definition and widefield imaging of the posterior segment, patient care will certainly improve.” ■
References
- Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012 Apr;32(4):785-791.
- Silva PS, Cavallerano JD, Haddad NM, Kwak H, Dyer KH, Omar AF, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949-956.
- Silva PS, Dela Cruz AJ, Ledesma MG, van Hemert J, Radwan A, Cavallerano JD, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465-2472.
- Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698.
- Heier JS. The effect of intravitreal aflibercept on capillary non-perfusion in patients with proliferative retinopathy and/or macular edema secondary to proliferative diabetic retinopathy and central retinal venous occlusive disease (ANDROID Study). Paper presentation; Macula Society annual meeting, Feb 26, 2016, Miami, FL.
- DRCR.net . Peripheral diabetic retinopathy (DR) lesions on ultrawide-field fundus images and risk of DR worsening over time. Available from: https://public.jaeb.org/drcrnet/stdy/239 ; last accessed Feb. 28, 2019.
- Gildea D. The diagnostic value of optical coherence tomography angiography in diabetic retinopathy: a systematic review. Int Ophthalmol. E-pub ahead of print: Oct. 31, 2018.
- Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45-50.
- Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina. 2015;35(11):2371-2376.
- Lin AD, Lee AY, Zhang Q, et al. Association between OCT-based microangiography perfusion indices and diabetic retinopathy severity. Br J Ophthalmol. 2017;101:960-964.
- Sambhav K, Abu-Amero KK, Chalam KV. Deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur J Ophthalmol. 2017;27(6):716-729.
- Cao D, Yang D, Huang Z, et al. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018;55(5):469-477.
- Carnevali A, Sacconi R, Corbelli E, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695-702.
- de Carlo TE, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364-2370.
- Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377-2383.
- Dupas B, Minvielle W, Bonnin S, et al. Association between vessel density and visual acuity in patients with diabetic retinopathy and poorly controlled type 1 diabetes. JAMA Ophthalmol. 2018;136(7):721-728.
- Verma A, Raman R, Vaitheeswaran K, et al. Does neuronal damage precede vascular damage in subjects with type 2 diabetes mellitus and having no clinical diabetic retinopathy? Ophthalmic Res. 2012;47(4):202-207.
- Van Dijk HW, Kok PH, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(7):3404-3409.
- Rodrigues EB, Urias MG, Penha FM, et al. Diabetes induces changes in neuroretina before retinal vessels: a spectral-domain optical coherence tomography study. Int J Retina Vitreous. 2015;1:4.
- Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19):E2655-2664.
- Vujosevic S, Muraca A, Alkabes M, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39(3):435-445.
- van Dijk HW, Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011;51(2):224-228.
- Shikari H, Lammer J, Cheney M. Foveal disorganization of retinal inner layers (DRIL) is highly associated with worse vision in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2014;55(13):4418.
- Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309-1316.
- Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmol. 2015;133(7):820-825.
- Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560-2570.
- Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202-208.
- Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32(3):563-568.
- Verma A, Nagpal M, Mehrotra N. In vivo assessment of choroid in diabetic retinopathy by enhanced depth imaging in spectral domain optical coherence tomography. Asia Pac J Ophthalmol (Phila). 2016;5(5):319-323.
- Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6017-6024.








