There has been persistent interest in seeking alternatives to radiation therapy for the conservative management of choroidal melanoma. The main issue with radiation therapy, despite high local control rate (better than 85%), is significant vision loss.1-3
Several modalities have been tried. Direct tumor photocoagulation has been largely abandoned because of lower local control rates.4-6 After initial enthusiasm,7,8 primary transpupillary thermotherapy is now used for limited indications because of concerns for recurrence (intraocular and extraocular) over the long term (Figure 1).9-11

Photodynamic therapy, effective for circumscribed choroidal hemangioma,12,13 has also been tried for the treatment of selected choroidal melanoma. Although initial reports over short-term follow-up (mean 28 months) had shown encouraging results (stable or regression in 83%),14 larger studies (n=36) report high local recurrence rates (30% to 38%).15-17 Even when only smaller tumors (n=12; less than 3.7 mm in thickness) and those that are amelanotic or lightly pigmented, are selected for treatment, the year 5 recurrence rate can be similarly high (33%).18
There is yet another attempt at achieving local control that is comparable to brachytherapy in carefully selected cases of small choroidal melanoma with light-activated therapy. AU-011 (Aura Biosciences) consists of synthetic, recombinantly derived viral-like particles (VLPs), derived from the human papillomavirus (HPV), that are conjugated to approximately 200 infrared-activated small molecules per VLP.19 The VLP bioconjugates bind selectively to uveal melanoma cells (and other solid tumor cells) that overexpress heparan sulfate proteoglycans.20 Upon activation with a 689-nm diode laser, the same used in photodynamic therapy, the small molecules selectively destroy the cell membrane of malignant cells (Figure 2).
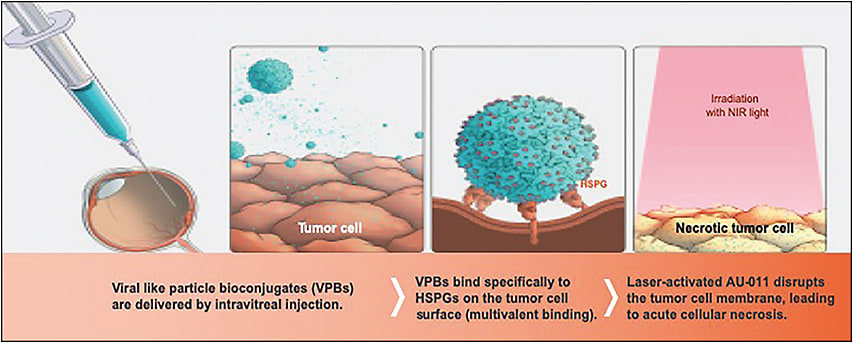
The 2-year interim results of a phase 1b/2 open-label clinical trial of AU-011 for the treatment of small to medium choroidal melanoma (NCT03052127) were recently presented at the 2019 meeting of the Association for Research in Vision and Ophthalmology in Vancouver, Canada.21 The selection criteria were based on clinical diagnosis of small to medium sized choroidal melanoma either with documented growth (thickness 1.2 mm to 3.4 mm and largest basal diameter [LBD] <16 mm) or with high risk of growth (thickness 2.0 mm to 3.4 mm and LBD <16 mm with subretinal fluid and orange pigment or visual changes or symptoms). The primary objective was to assess for safety (drug or treatment-related adverse events) and the secondary objective was to determine local tumor control (preliminary efficacy), visual acuity preservation, and immunogenicity (at 3 months).
This is an open-label, multicenter, ascending single and repeat dose and cycle study. Study cohorts include dosing with intravitreal AU-011 at 3 dose levels (20 μg, 40 μg, or 80 μg), single and repeat dose regimens and cycles, followed by light activation with 1 or 2 laser applications of 689-nm laser at a fluence of 50 J/cm2. A second dose expansion cohort is currently enrolling subjects to be treated with 2 cycles of AU-011 and also subjects in a prospective natural history (observation) cohort (Figure 3).

Overall, single and multiple administrations of AU-011 were well tolerated. Mild and moderate adverse events that included intraocular inflammation and increased intraocular pressure could be adequately managed. From the total cohort of 36, 12 patients in the single-dose cohort demonstrated a modest tumor control rate of 67% with a follow-up period of 9 to 24 months, and 22 patients in the multiple-dose cohort (2 patients lost to follow-up) demonstrated a modest tumor control rate of 77% with a follow-up period of 0.5 to 18 months. Subjects treated with the maximum safe and tolerated dose (80 µg with 2 lasers) with 0.5 months to 6 months follow-up have a tumor control rate of 92% (13 of 14 subjects). Vision was preserved in all patients at 3 months or longer up to 24 months. As the phase 1/2 studies come to completion, Aura Biosciences is planning 2 identical phase 3 trials comparing treatment with 2 doses of light-activated AU-011 vs sham treatment in subjects with primary high-risk indeterminate lesions or small choroidal melanoma. The long-term results of tumor control will be of great interest as we seek less toxic but equally effective therapeutic alternatives to radiation therapy for choroidal melanoma. RP
REFERENCES
- Bellerive C, Aziz HA, Bena J, et al. Local failure after episcleral brachytherapy for posterior uveal melanoma: patterns, risk factors, and management. Am J Ophthalmol. 2017;177:9-16.
- Echegaray JJ, Bechrakis NE, Singh N, Bellerive C, Singh AD. Iodine-125 brachytherapy for uveal melanoma: a systematic review of radiation dose. Ocul Oncol Pathol. 2017;3(3):193-198.
- Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostication tool. JAMA Ophthalmol. 2016;134(6):615-620.
- Meyer-Schwickerath G, Vogel M. Treatment of malignant melanomas of the choroid by photocoagulation. Trans Ophthalmol Soc U K. 1977;97(3):416-420.
- Orellana J, McPherson AR. Xenon-arc photocoagulation of medium-sized malignant melanomas. Ann Ophthalmol. 1984;16(5):412-416.
- Shields JA, Glazer LC, Mieler WF, Shields CL, Gottlieb MS. Comparison of xenon arc and argon laser photocoagulation in the treatment of choroidal melanomas. Am J Ophthalmol. 1990;109(6):647-655.
- Oosterhuis JA, Journee-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995;113(3):315-321.
- Robertson DM, Buettner H, Bennett SR. Transpupillary thermotherapy as primary treatment for small choroidal melanomas. Trans Am Ophthalmol Soc. 1999;97:407-434.
- Robertson DM. Small choroidal melanomas treated with transpupillary thermotherapy and cryotherapy. Arch Ophthalmol. 2008;126(8):1156-1157.
- Singh AD, Kivela T, Seregard S, Robertson D, Bena JF. Primary transpupillary thermotherapy of “small” choroidal melanoma: is it safe? Br J Ophthalmol. 2008;92(6):727-728.
- Mashayekhi A, Shields CL, Rishi P, et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: importance of risk factors in tumor control. Ophthalmology. 2015;122(3):600-609.
- Singh AD, Kaiser PK, Sears JE. Choroidal hemangioma. Ophthalmol Clin North Am. 2005;18(1):151-161, ix.
- Michels S, Michels R, Beckendorf A, Schmidt-Erfurth U. [Photodynamic therapy for choroidal hemangioma. Long-term results]. Ophthalmologe. 2004;101(6):569-575.
- Rundle P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol. 2014;98(4):494-497.
- Fabian ID, Stacey AW, Harby LA, Arora AK, Sagoo MS, Cohen VML. Primary photodynamic therapy with verteporfin for pigmented posterior pole cT1a choroidal melanoma: a 3-year retrospective analysis. Br J Ophthalmol. 2018;102(12):1705-1710.
- O’Day RF, Pejnovic TM, Isaacs T, Muecke JS, Glasson WJ, Campbell WG. Australian and New Zealand study of photodynamic therapy in choroidal amelanotic melanoma. Retina. 2019 Mar 19. [Epub ahead of print]
- Jmor F, Hussain RN, Damato BE, Heimann H. Photodynamic therapy as initial treatment for small choroidal melanomas. Photodiagnosis Photodyn Ther. 2017;20:175-181.
- Tran HV, Schalenbourg A, Zografos L. Bilateral circumscribed choroidal hemangioma in an otherwise healthy individual. Retin Cases Brief Rep. 2007;1(3):149-152.
- Aura Biosciences website. http://www.aurabiosciences.com/about-us .
- Kines RC, Cerio RJ, Roberts JN, et al. Human papillomavirus capsids preferentially bind and infect tumor cells. Int J Cancer. 2016;138(4):901-911.
- McCannel TA, Bhavsar A, Capone A, et al. Two year results of a phase 1b/2 open-label clinical trial of AU-011 for the treatment of small to medium choroidal melanoma. Paper presented at: the 2019 annual meeting of the Association for Research in Vision and Ophthalmology; Vancouver, Canada.








