EDITED BY SUNIL SRIVASTAVA, MD
Cancer therapeutics have progressed rapidly to target the mechanisms by which malignant cells can evade detection by the immune system. Some of the newest therapeutic agents have been strongly associated with adverse events related to immune dysregulation, otherwise known as immune-related adverse events (irAEs). Uveitis is on the list of systemic irAEs that occur as a result of these medications, and the manifestations of uveitis that occur are manifold and variable. Here, we briefly review the newer agents that have been implicated in cancer drug-induced uveitis, including immune-checkpoint inhibitors and BRAF and MEK inhibitors.1,2
IMMUNE-CHECKPOINT INHIBITORS
Immune-checkpoint inhibitors have emerged as a novel way of targeting certain malignancies. These medications work by activating normally suppressed T-cell function, leading to upregulation of the immune state in order to combat cancers. Cytotoxic T lymphocyte antigen 4 (CTLA-4) and program death protein 1 (PD-1) are receptors located directly on the T cell that normally suppress T-cell activity when activated; medications designed to block these receptors upregulate T-cell activity. Similarly, programmed death ligand 1 (PD-L1) is a receptor located directly on cancer cells that normally interacts with PD-1 to suppress the immune system and can also be blocked to upregulate T cells. Specific inhibitors have been developed for all 3 targets and have shown promise in cancer therapeutics; however, the upregulation of the immune system caused by these targeted inhibitors may lead to unwanted side effects of intraocular inflammation.1,2
Cyrus Golshani, MD, is a medical retina and uveitis fellow at the Cole Eye Institute of Cleveland Clinic in Cleveland, Ohio. Arthi Venkat, MD, is a medical retina and uveitis staff physician at the Cole Eye Institute of Cleveland Clinic in Cleveland, Ohio. The authors report no related disclosures. Reach Dr. Venkat at venkata@ccf.org.
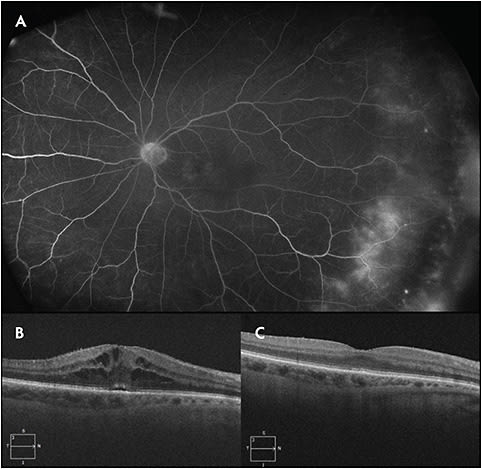
Immune checkpoint inhibitors lead to various presentations of uveitis. Ipilimumab, a CTLA-4 inhibitor, has been reported to cause mild cases of anterior uveitis as well as severe panuveitis.2 Monoclonal antibody inhibitors to PD-1 (nivolumab and pembrolizumab) and PD-L1 (atezolizumab) similarly activate the body’s immune response and can lead to manifestations of uveitis ranging from mild anterior uveitis with macular edema to a Vogt-Koyanagi-Harada (VKH)-like syndrome.3,4 Although some scenarios necessitate systemic corticosteroids, many case reports have found success in controlling and improving the intraocular inflammation with local therapy alone and without cessation of cancer therapy.2 Figure 1 demonstrates a case of a 73-year-old female with stage 3A squamous-cell lung cancer on atezolizumab who developed cystoid macular edema in the right eye on spectral domain optical coherence tomography (SD-OCT). Fluorescein angiography (FA) showed macular and peripheral leakage in both eyes. She received a single injection of 2 mg intravitreal triamcinolone acetonide in the right eye and was treated with topical difluprednate 4 times a day in the left eye which led to complete resolution of her posterior uveitis and allowed her to continue cancer therapy.
BRAF/MEK INHIBITORS
Certain human cancers have been associated with dysregulation of the mitogen-activated protein kinase (MAPK) signaling pathway.5 Specifically within this pathway, mitogen-activated protein kinase kinase (MEK) and V-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations have been shown to lead to cell proliferation and cancer development. Most notably, BRAF mutations have been found in more than 50% of melanomas.6 Thus, targeted therapies aimed at inhibiting the MAPK pathway have recently been developed, such as MEK and BRAF inhibitors. Examples of MEK inhibitors include trametinib and binimetinib, and BRAF inhibitors include vemurafenib and dabrafenib. Although these targeted medications have been effective at treating cancers that were previously not amenable to classic chemotherapy, they have all been reported to potentially cause cancer-drug–induced uveitis.6
Similar to immune-checkpoint inhibitors, BRAF and MEK inhibitors also cause a number of uveitis manifestations. Vemurafenib has been reported to cause intraocular inflammation ranging from low-grade anterior uveitis to severe explosive panuveitis complicated by serous retinal detachment.7 One report found bilateral intermediate uveitis that developed a few weeks after starting both dabrafenib and trametinib.8 Similarly, another case report in a patient taking the same 2 medications developed severe bilateral panuveitis with multiple serous retinal detachments resembling VKH disease. Eventually the intraocular inflammation resolved completely with discontinuation of the drug and topical steroid therapy.9 Figure 2 shows a patient on BRAK/MEK inhibitor therapy for metastatic melanoma with 360 choroidal detachments in both eyes.

IMPORTANCE OF IMAGING
In addition to conducting a thorough history and review of a patient’s medications, the importance of obtaining imaging for patients suspected of having cancer drug-induced uveitis cannot be overstated. Multimodal imaging may identify subclinical posterior-segment inflammation in some cases. Figure 3 highlights a case of a 51-year-old female with a history of melanoma complicated by lymph node metastasis recently started on nivolumab therapy. Shortly after initiating nivolumab, she began noticing “hazy vision” in both eyes. On examination, she had grade 1+ anterior-chamber cell with posterior synechiae in both eyes despite frequent topical prednisolone acetate, and there was no obvious intermediate or posterior uveitis seen clinically. Although OCT did not demonstrate macular edema, an FA was obtained on the basis of the persistent inflammation, which revealed leakage of the disc and periphery. The patient was switched to topical difluprednate therapy that resolved the patient’s symptoms and the angiographic leakage.

CONCLUSION
Novel targeted therapies with BRAF/MEK inhibitors and immune-checkpoint inhibitors have emerged as effective treatments for certain cancers. Retinal physicians must be aware of these newer agents and their potential and variable ophthalmic inflammatory side effects. Imaging with OCT and FA can be useful for recalcitrant anterior-chamber inflammation secondary to these agents, because subclinical posterior-segment inflammation can be identified. Although cessation of these agents usually leads to resolution of uveitis, local therapy has shown success and allows patients to continue these life-saving therapies while maintaining good quality of life. RP
REFERENCES
- Moorthy RS, Moorthy MS, Cunningham ET. Drug-induced uveitis. Curr Opin Ophtalmol. 2018;29(6):588-603.
- Dalvin LA, Shields CL, Orloff M, et al. Checkpoint inhibitor immune therapy. Retina. 2018;38(6):1063-1078.
- De Velasco G, Bermas B, Choueiri TK. Autoimmune arthropathy and uveitis as complications of programmed death 1 inhibitor treatment. Arthritis Rheumatol. 2016;68:556-557
- Arai T, Harada K, Usui Y, et al. Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol. 2017;44:975-976
- Akinkleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27.
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
- Guedj M, Queant A, Funck-Brentano E, et al. Uveitis in patients with late-stage cutaneous melanoma treated with vemurafenib. JAMA Ophthalmol. 2014;132:1421-1425.
- Joshi K, Karydis A, Gemenetzi M, et al. Uveitis as a result of MAP kinase pathway inhibition. Case Rep Ophthalmol. 2013;4:279-282.
- Draganova D, Kerger J, Caspers L, et al. Severe bilateral panuveitis during melanoma treatment by dabrafenib and trametinib. J Ophthalmic Inflamm Infect. 2015;5:17-19.








