The contemporary care of a patient with an intraocular tumor requires advanced, multimodal ophthalmic imaging.1 The most intuitive purpose of this imaging is to document the status of the lesion and its secondary effects so that it may be monitored over time to detect change. However, imaging may also provide diagnostic information that is not afforded by ophthalmoscopy alone. Fortunately, most imaging modalities used in the practice of ocular oncology are eminently familiar to the retina specialist.
FUNDUS DRAWING
Before the popularization of fundus photography, detailed retinal drawings served the primary role of documenting the ophthalmoscopic features of intraocular tumors (Figure 1). Fundus drawing may record some ophthalmoscopic features in higher fidelity than fundus photography, such as subtle subretinal fluid overlying a tumor or peripheral findings that cannot be captured by the camera. However, for many ocular oncologists, the time required to produce an outstanding fundus diagram is prohibitive, and most ocular oncologists instead use fundus photography to document ophthalmoscopic features for many patients. It should be noted that fundus drawings remain essential for children with retinoblastoma because fundus photography under anesthesia (as described further below) often fails to capture certain tumor details (especially in peripheral tumors) and cannot always reliably capture the relative locations of the often multiple tumors within an eye.

PHOTOGRAPHY
Photography is the most essential imaging modality in ocular oncology, with fundus cameras being the primary method by which to photograph the posterior segment. Fundus cameras may be divided into widefield and traditional groups, each with advantages and disadvantages.
Standard-Field Fundus Photography
Fundus cameras are available in most ophthalmology clinics and have the advantage of providing images that can be very accurate depictions of what is visualized ophthalmoscopically. The images are often of very high resolution and reveal “true” color, which can be very important when documenting fundus lesions accurately (Figure 2). However, the disadvantages of standard fundus cameras are significant. First, they require significant expertise to use properly. Standard fundus images that are not focused correctly are often of such poor quality that they have limited clinical use. Second, media opacity such as cataract and corneal scarring generally impairs the quality of images from standard fundus cameras to a greater degree than widefield cameras. Finally, standard fundus cameras usually include only a relatively narrow capture angle of 30° to 50° — this is particularly bothersome for peripheral choroidal or retinal tumors, which sometimes cannot be captured with this imaging modality.
Widefield Fundus Photography
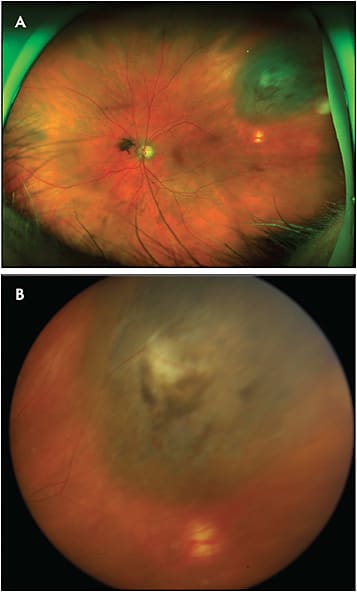
The primary advantage of widefield fundus cameras compared to their traditional counterparts is their wider capture angle. This often allows documentation of the entire base of an intraocular lesion and its location relative to the posterior pole, usually with a single shot (Figure 3). In addition, contemporary widefield cameras are technically very easy to use, requiring little training to produce outstanding photos reliably. Moreover, the quality of widefield fundus images is not usually degraded as much by media opacity as it is with standard fundus images. Because of these advantages, widefield imaging has become the standard at most ocular oncology practices.
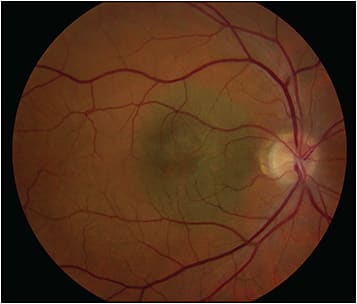
The Optos widefield fundus cameras are the most popular in the field of ocular oncology. They are simple to use and produce 200-degree fundus images (sometimes called ultrawidefield) with relative ease. The main disadvantage of the Optos camera is that it produces false color images that often do not reliably represent the true color seen with ophthalmoscopy. This can be particularly bothersome when imaging a choroidal hemangioma, because its salient diagnostic feature is its color (red/orange) (Figure 4). An alternative widefield fundus camera is the Clarus (Carl Zeiss Meditec AG), which has a narrower imaging field than Optos but produces images in true color.
Anterior-Segment Photography
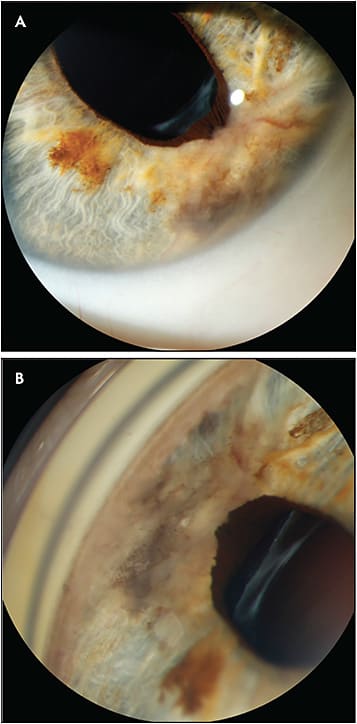
The slit-lamp camera (with or without the use of a gonioscopic lens) is primarily used to document tumors that involve the conjunctiva, cornea, anterior chamber, iris, iridocorneal angle, and ciliary body, as well as tumors that secondarily involve these structures, so that they can be monitored over time (Figure 5). Secondary tumor effects such as pseudohypopyon, iris neovascularization, and sentinel scleral vessels can also be readily documented by this modality. Despite its name, the “slit” function of the light source need not be employed for most anterior segment lesions, but can sometimes be useful for documenting the depth of a cornea-involving tumor or the distance of an anterior intraocular tumor to the cornea.
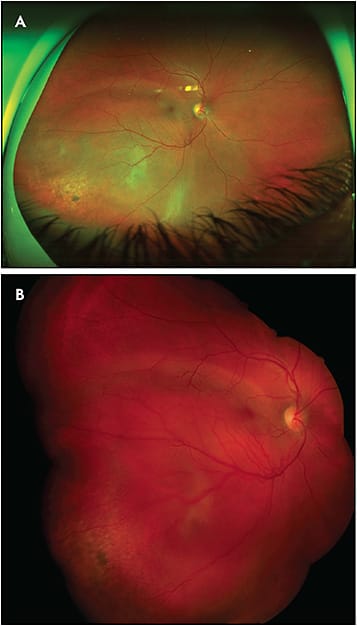
Handheld Contact Fundus Cameras
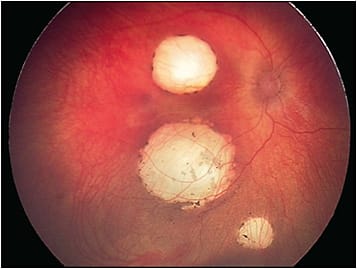
The RetCam (Natus Medical Incorporated) is a handheld camera that produces high-resolution fundus images up to 130 degrees (Figure 6). Images of the anterior segment and iridocorneal angle can also be captured. The RetCam can be paired with a filter to produce fluorescein angiography images as well. The RetCam requires contact with the cornea and, in the field of ocular oncology, is primarily used to image pediatric patients with conditions such as intraocular tumors (eg, retinoblastoma, astrocytic hamartomas) and tumor-simulating conditions (eg, Coats disease).
Fundus Autofluoresence
Lipofuscin is a byproduct of phagocytosis of retinal photoreceptor outer segments by the retinal pigment epithelium (RPE) and is associated with oxidative stress.2 Many diseases cause accumulation of this substance in and around the RPE, and choroidal melanoma is a notable one. Lipofuscin fluoresces with a specific emission profile when stimulated with light, a phenomenon known as fundus autofluorescence (FAF). Kitagawa et al first quantified this property of lipofuscin in 1989 using fluorophotometry.3 Fundus autofluoresence is a type of fundus photography that exploits this quality of lipofuscin and may be performed by a fundus camera or a confocal scanning laser ophthalmoscope.
Lipofuscin is often gray or orange overlying choroidal melanoma that are amelanotic or pigmented, respectively. However, all lipofuscin tends to appear bright on FAF photography images. In the practice of ocular oncology, the primary use of autofluoresence images is to confirm that suspicious pigment seen overlying a suspected choroidal melanoma is indeed lipofuscin (and not drusen, which tend to appear more well circumscribed and less bright on FAF images). A secondary use of FAF, especially when paired with an ultrawidefield camera, is to reveal the hyperautofluoresence often associated with tumor-related subretinal fluid and exudative retinal detachments, which are usually gravity dependent (Figure 7). Notably, near-infrared fundus autofluoresence imaging highlights melanin more than lipofuscin but it not routinely used in ocular oncology.

INTRAVENOUS ANGIOGRAPHY
Fluorescein Angiography
First developed by Novotnoy and Alvis in 1959, fluorescein angiography (FA) paired specialized fundus photography with intravenous infusion of sodium fluorescein to examine the retinal and choroidal circulation.4 Soon after that time, FA of the iris was developed.5 Today, FA can be performed with both traditional and ultrawidefield cameras.
The most high-yield use of FA in ocular oncology is to detect small retinal hemangioblastomas in Von Hippel-Lindau syndrome, particularly using ultrawidefield FA. Patients with this condition often have many small retinal tumors that are difficult to detect ophthalmoscopically but that usually fluoresce brightly on FA. If left untreated, these lesions tend to grow and can be quite difficult to treat later. Fluorescein angiography can also be useful occasionally in the management of radiation retinopathy. Similar to the case of diabetic retinopathy, leaking microaneurysms, retinal or optic nerve neovascularization, and areas of retinal nonperfusion may be identified by FA, although the vast majority of radiation retinopathy and optic neuropathy is managed easily and appropriately without FA.
Other than for the aforementioned purposes, the use of FA in ocular oncology may be less important than traditionally considered. For example, although the leakage identified by FA may be characteristic for choroidal melanoma, tumor leakage may be readily observed by detecting subretinal fluid on OCT, a modality that is significantly less cumbersome and invasive, as described below.
The classically described FA feature of “double circulation” associated with choroidal melanoma is actually seen in less than 60% of cases and can be detected by ultrasonography (as described below) or via the frank presence of intratumoral vessels seen on ophthalmoscopy. The use of iris FA is primarily limited to vascular and other rare tumors of the iris that cannot be readily diagnosed by alternative methods.
Indocyanine Green Angiography
The choroidal vasculature is relatively impermeable to indocyanine green, which permits indocyanine green angiography (along with the deeper penetration afforded by the infrared light source) to outline the choroidal circulation in greater detail than is possible with FA. Indocyanine green angiography is of particular use in the diagnosis of choroidal hemangioma by showing characteristic features including early diffuse hypercyanescence or a late “dye washout” with central hypocyanescence with a surrounding hypercyanescent rim.6,7 However, most choroidal hemangiomas are diagnosed without this imaging modality based on their characteristic appearance on ophthalmoscopy.
ULTRASONOGRAPHY
B-scan ultrasound can be subdivided into posterior B-scan ultrasound and anterior B-scan ultrasound, the latter of which is commonly called ultrasound biomicroscopy or UBM. Both methods are absolutely essential for the practice of ocular oncology for 2 primary reasons: (1) examination of a nontransparent tumor using an optical system (ie, slit-lamp or ophthalmoscopy) cannot reliably discern its thickness, and (2) tumors of the anterior choroid and ciliary body, as well as extrascleral or retrolaminar extension of a posterior intraocular mass, cannot often be completely examined directly because their overlying sclera blocks the transmission of light.
Posterior B-Scan
Posterior B-scan ultrasound usually employs frequencies of 10 MHz to 20 MHz. Aside from the size and shape of intraocular tumors, B-scan ultrasound may assist the ocular oncologist in identifying the internal acoustic characteristics of a lesion (intensity and relative heterogeneity of internal reflectivity, intratumoral cysts/calcium, and presence of internal vascularity) and help quantify associated features such as retinal detachment. In addition, posterior B-scan can be used to evaluate tumor features when significant media opacity exist, such as cataract, vitreous hemorrhage, or massive retinal detachment. Importantly, posterior B-scan is often used when placing episcleral radioactive plaques to confirm proper placement in relation to an intraocular tumor. Although only present in a minority of large tumors, a “collar-stud” shape on posterior B-scan is nearly pathognomonic for uveal melanoma, although uveal metastases may also rarely present with this morphology.
Anterior B-Scan
Anterior B-scan (UBM) uses higher frequencies than posterior B-scan (35 MHz or more), and these frequencies permit higher scan resolution but have a decreased depth of penetration. Anterior B-scan is most useful for examining tumors involving the iris, iridocorneal angle, ciliary body, and anterior choroid, as well as lesions of the lens (including an intraocular lens or Sommering ring cataract). With anterior B-scan, the oscillating tip of the probe may be placed on a water bath or a fluid-filled balloon tip, the latter being much more user friendly. The techniques required for anterior B-scan are very similar to those of posterior B-scan, but they are less familiar to ophthalmologists, including retina specialists.
A-Scan
Using a single “beam” of sound, this type of ultrasound can quantify the thickness and internal reflectivity of tumors as well as the axial length of the globe. Accurate axial length measurements are essential in 3-dimensional treatment planning for proton-beam radiotherapy of ocular tumors. Although tumor thickness can be measured with A-scan, this technique is less preferred and may be inaccurate, because without concomitant 2-dimensional morphologic imaging data, it can be difficult to confirm that the thickest part of the tumor is being measured.
Acoustic “internal reflectivity” is a quantitative measure of the intensity of ultrasound echoes within a substance. Tumors containing calcium, such as retinoblastoma and choroidal osteoma, may create an acoustic “spike” with high surface reflectivity, whereas cysts will have a very low internal reflectivity. Uveal melanomas are classically found to be of “low” or “decrescendo” internal reflectivity, although this is variable, depends on tumor pigment and thickness, and cannot be determined accurately for thin lesions. Although it is traditionally taught that internal reflectivity may only be measured by A-scan, posterior B-scan may also be used and displays internal reflectivity as signal brightness. Diagnostic A-scans usually require a standardized probe wherein the surface spike of the retina is calibrated at 100%.
OPTICAL COHERENCE TOMOGRAPHY
Standard Optical Coherence Tomography
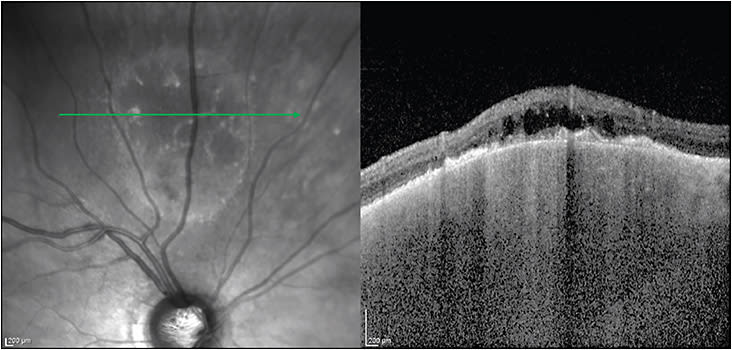
“Standard” OCT refers to the most common form of this imaging modality and obtains cross-sectional images from a seated patient. Since its development, OCT has played a progressively larger role in the way we as retina specialists diagnose and manage cases of all types — intraocular tumors included. Although a comprehensive picture of the ways in which OCT is utilized in ocular oncology is beyond the scope of this summary, there are several notable highlights. When imaging choroidal tumors, the primary purpose of OCT is to evaluate for overlying subretinal fluid that is commonly found with choroidal melanomas, metastasis, and symptomatic choroidal hemangiomas (among other lesions) and is less common in choroidal nevi. OCT also reveals signs of chronicity in choroidal nevi, such as overlying drusen, RPE thickening, and outer retinal atrophy (Figure 8). OCT can also clinch the diagnosis of sub-RPE hemorrhage or fluid simulating a melanoma when a sharp elevation in the RPE is seen and, sometimes, a few pixels of intact choriocapillaris (ie, no choroidal mass) can also be seen at the edge of the pigment epithelial detachment. OCT may reveal characteristic findings in several intraocular tumors such as astrocytic hamartomas (“moth-eaten” spaces8), vitreoretinal-involving lymphoma (subretinal/RPE deposits9), and choroidal osteoma (characteristic bone-like morphology10). In thinner tumors, especially those lacking pigment, enhanced-depth imaging (or “EDI”) OCT may be able to capture their entire thickness, enabling measurement that may be more accurate than ultrasound (Figure 9). OCT is essential in the management of patients with macular edema secondary to intraocular tumors or radiation retinopathy.

Optical Coherence Tomography Angiography
Optical coherence tomography angiography (OCTA) is a noninvasive angiographic technique that has been gaining popularity among ocular oncologists. It has been used to evaluate the retinal and choroidal vascular signal in cases of intraocular tumors before and after treatment, as well as to evaluate radiation retinopathy. OCTA appears to show characteristic signals in certain tumors,11-13 as well as specific changes following ocular radiation or chemotherapy.14,15 For now, further investigation of this emerging imaging modality is needed to determine its role in the diagnosis or monitoring of intraocular tumors.
Handheld Optical Coherence Tomography
Handheld OCT is most useful for examining patients who cannot position at a standard seated OCT device — usually children. Handheld OCT under anesthesia is useful in the management of several types of pediatric tumors, especially in retinoblastoma where clinically invisible tumors and recurrences may be detected.16,17 Handheld OCT is also useful to evaluate the macular structure of patients with macula-involving retinoblastoma.
CONCLUSION
Imaging in ocular oncology is essential and must be multimodal. Contemporary imaging techniques permit the recognition, documentation and identification of ocular tumors and their secondary effects. Mastery of these techniques is indispensable in the management of these tumors. RP
REFERENCES
- Seider MI, Damato BE. Imaging the intraocular tumor. Expert Rev Ophthalmol. 2014;9(5):387-399.
- Nilsson SE, Sundelin SP, Wihlmark U, Brunk UT. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol. 2003;106(1):13-16.
- Kitagawa K, Nishida S, Ogura Y. In vivo quantitation of autofluorescence in human retinal pigment epithelium. Ophthalmologica. 1989;199(2-3):116-121.
- Novotny HR, Alvis D. A method of photographing fluorescence in circulating blood of the human eye. Tech Doc Rep SAMTDR USAF Sch Aerosp Med. 1960;60-82:1-4.
- Vannas A. Fluorescein angiography of the vessels of the iris in pseudoexfoliation of the lens capsule, capsular glaucoma and some other forms of glaucoma. Acta Ophthalmol Suppl. 1969;105:1-75.
- Schalenbourg A, Piguet B, Zografos L. Indocyanine green angiographic findings in choroidal hemangiomas: A study of 75 cases. Ophthalmologica. 2000;214(4):246-252.
- Arevalo JF, Shields CL, Shields JA, Hykin PG, De Potter P. Circumscribed choroidal hemangioma: characteristic features with indocyanine green videoangiography. Ophthalmology. 2000;107(2):344-350.
- Shields CL, Say EAT, Fuller T, Arora S, Samara WA, Shields JA. Retinal astrocytic hamartoma arises in nerve fiber layer and shows “moth-eaten” optically empty spaces on optical coherence tomography. Ophthalmology. 2016;123(8):1809-1816.
- Barry RJ, Tasiopoulou A, Murray PI, et al. Characteristic optical coherence tomography findings in patients with primary vitreoretinal lymphoma: a novel aid to early diagnosis. Br J Ophthalmol. 2018;102(10):1362-1366.
- Shields CL, Arepalli S, Atalay HT, Ferenczy SR, Fulco E, Shields JA. Choroidal osteoma shows bone lamella and vascular channels on enhanced depth imaging optical coherence tomography in 15 eyes. Retina. 2015;35(4):750-757.
- Pellegrini M, Corvi F, Invernizzi A, Ravera V, Cereda MG, Staurenghi G. Swept-source optical coherence tomography angiography in choroidal melanoma: an analysis of 22 consecutive cases. Retina. 2018. [Epub ahead of print]
- Sagar P, Rajesh R, Shanmugam M, Konana VK, Mishra D. Comparison of optical coherence tomography angiography and fundus fluorescein angiography features of retinal capillary hemangioblastoma. Indian J Ophthalmol. 2018;66(6):872-876.
- Cennamo G, Romano MR, Breve MA, Velotti N, Reibaldi M, de Crecchio G. Evaluation of choroidal tumors with optical coherence tomography: enhanced depth imaging and OCT-angiography features. Eye (Lond). 2017;31(6):906-915.
- Shields CL, Say EA, Samara WA, Khoo CT, Mashayekhi A, Shields JA. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma: comparison of irradiated versus nonirradiated eyes in 65 patients. Retina. 2016;36(8):1493-1505.
- Sioufi K, Say EAT, Ferenczy SC, Leahey AM, Shields CL. Optical coherence tomography angiography findings of deep capillary plexus microischemia after intravenous chemotherapy for retinoblastoma. Retina. 2017. [Epub ahead of print]
- Cao C, Markovitz M, Ferenczy S, Shields CL. Hand-held spectral-domain optical coherence tomography of small macular retinoblastoma in infants before and after chemotherapy. J Pediatr Ophthalmol Strabismus. 2014;51(4):230-234.
- Seider MI, Grewal DS, Mruthyunjaya P. Portable optical coherence tomography detection or confirmation of ophthalmoscopically invisible or indeterminate active retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):965-968.








