The management of neovascular age-related macular degeneration (nAMD) has changed dramatically over the last 10 years. The MARINA trial1 showed that monthly administration of ranibizumab for 2 years not only prevented vision loss but on average the visual acuity in treated eyes improved by around 1 to 2 lines on the ETDRS chart over this time frame. In addition, the ANCHOR trial2 confirmed the vastly superior functional outcome of ranibizumab when compared to photodynamic therapy (PDT), which was the accepted treatment for the subtype of predominantly classic nAMD at that time. Subsequently, the VIEW 1 and VIEW 2 studies showed similar benefits for aflibercept, and demonstrated that bimonthly injections of aflibercept were noninferior to monthly injections of ranibizumab.3
Currently, 3 anti-VEGF drugs are in use for nAMD. Two of them, ranibizumab (Lucentis; Genentech) and aflibercept (Eylea; Regeneron), are approved for this indication. The third, bevacizumab (Avastin; Genentech), is used off label for nAMD, and is a cheaper, more cost-effective treatment.4 The efficacy of the 3 treatments has been shown in numerous studies.
TREATMENT REGIMENS IN NEOVASCULAR AMD
The MARINA and ANCHOR trials revealed the superior efficacy of ranibizumab compared to no treatment for occult and minimally classic choroidal neovascularization (MARINA) and compared to PDT in predominantly classic choroidal neovascularization (ANCHOR) when administered monthly to eyes with nAMD. Subsequently, the PrONTO study5 demonstrated the efficacy of administering anti-VEGF drugs using a pro re nata (PRN) regimen. In this highly influential trial, patients were treated over 3 consecutive months with intravitreal injections of ranibizumab (loading phase) followed by a PRN regimen. Patients were seen monthly but retreatment was performed only if specific criteria were met (and these included a loss of 5 letters of VA, an increase in retinal thickness, or an increase in retinal fluid). Despite a decrease in the number of treatments (only 9.9 intravitreal injections were administered on average over 24 months), the mean improvement in visual acuity at month 24 was 11.1 letters, which was comparable to that achieved in the trials where treatment was administered every month over a similar time period.
Subsequently, several large trials that tested different drugs combined with reduced frequency treatment regimens were undertaken. In the CATT study, 1,107 patients were assigned to 4 treatment groups defined by drug (ranibizumab or bevacizumab) and by dosing regimen (monthly or PRN).6 At 1 year, patients initially assigned to monthly treatment were reassigned randomly to either monthly or PRN treatment. After 2 years, the mean gain in visual acuity was similar for both drugs among patients following the same regimen, with a marginally greater gain for monthly than for PRN treatment (8.8 letters for monthly ranibizumab and 7.8 for monthly bevacizumab, 6.7 for PRN ranibizumab and 5.0 for PRN bevacizumab). A switch from monthly to PRN treatment resulted in greater mean decrease in vision during year 2 and a lower proportion of eyes without signs of lesion activity.
The IVAN trial employed a factorial design with participants with nAMD randomized to 1 of 2 drugs (ranibizumab or bevacizumab) and 1 of 2 regimens (monthly or discontinuous).7 Participants in the discontinuous treatment arm could have their treatment withheld on completion of the 3-month loading phase. However, if any signs of lesion activity were detected on clinical examination or on OCT, treatment was recommenced but was delivered in blocks of 3 (ie, a repeat of the loading phase). After 2 years, bevacizumab and PRN treatment were neither noninferior nor inferior to ranibizumab or monthly treatment, and BCVA gains were similar in the drug comparisons as well as in the regimen comparisons. Interestingly, secondary functional measures that included near acuity and reading speed showed marginally worse outcomes for the discontinuous treatment arm.
Later studies also demonstrated efficacy for ranibizumab when given in a treat-and-extend (T&E) regimen. For example, the TREX-AMD study compared efficacy between monthly ranibizumab injections and injections according to a T&E protocol.8 The T&E patients were treated monthly for at least 3 doses until resolution of clinical and spectral-domain optical coherence tomography (SD-OCT) evidence of disease activity. When resolution, which was defined as a fluid-free macula, was achieved, the interval between visits was individualized according to a strict protocol. This involved the extension of the interval between retreatments by 2-week increments (up to 12 weeks) if at every visit there was no evidence of disease activity. If at any visit OCT signs of disease activity were detected, the retreatment interval was reduced back incrementally to 4 weeks. The average number of treatments at 12 months in the T&E arm was 10.1 vs 13.0 in the monthly cohort. Correspondingly, mean best-corrected visual acuity (BCVA) was 10.5 letters in the T&E cohort, and this was almost identical to the gains in the monthly treatment cohort.
RANDOMIZED TRIALS VS REAL-WORLD EVIDENCE
Despite the finding of clear benefit with an average of around 2 lines of visual improvement over baseline in almost all of the clinical trials,9 later real-world data sets show a disturbing decline in visual acuity gains over time. The AURA study, a multicenter study conducted in several countries (Canada, France, Germany, Ireland, Italy, the Netherlands, the United Kingdom, and Venezuela) and published in 2014, presented results based on real-life experience.10 In 2 years of follow-up, a different trend was noticed than was seen in prospective clinical trials: while visual acuity improved in the initial phase until about day 120, thereafter the gains were not maintained. The mean change in visual acuity from baseline to year 1 was +2.4, but only to +0.6 letters at year 2. In the long-term follow-up of participants who were enrolled in the CATT study, at 5 years the mean change in visual acuity was -3 letters from baseline and -11 letters from 2 years.11
Similar reductions in VA of around 2 lines or 10 ETDRS letters have been reported in the HORIZON study, which was an extension after 1 year of participation in the 2 pivotal ranibizumab clinical trials.12 In the second year, retreatment was at the investigator’s discretion with no definition of specific retreatment criteria. At 2 years, the mean change in BCVA relative to the initial study baseline was +2.0, and -11.8 in patients who were originally randomized to control who crossed over to receive ranibizumab. These findings suggest that the 2-line gains that were seen in the first year in the originally drug exposed group were lost and that the vision lost in the control group could not be recovered. These data are not surprising, because in untreated neovascular AMD, there is on average a 15-letter loss over a 1-year period, and instituting treatment after a year’s delay does not have the potential to overcome the lost VA. Furthermore, data exist to show that atrophy and fibrosis in the nAMD lesion occur rapidly when not treated, leading to permanent vision loss.13
The SEVEN-UP study assessed long-term outcomes (7-8 years) in the original participants of the MARINA and ANCHOR studies.14 At a mean of 7.3 years after entry into these studies, 43% of study eyes had a stable or improved letter score (≥0-letter gain) compared with the baseline. However, 34% declined by 15 letters or more, with an overall average decline of 8.6 letters. Thus, the data from long-term follow-up of participants enrolled in key randomized controlled clinical trials indicate a trend of reducing visual acuity over time. These data are also supported by several real-world datasets with very large numbers of patients. The Neovascular Age-Related Macular Degeneration Database analyzed data from 92,976 injections of ranibizumab conducted in 14 centers in the United Kingdom.15 Visual acuity gain was only 2 letters at year 1 and 1 letter at year 2. At year 3 there was a loss of 2 letters. Another real-world observational study presented the outcomes of more than 1,200 eyes with more than 5 years of follow-up from the Fight Retinal Blindness (FRB) database in Australia.16 As with previous studies, there was an initial gain of 6.3 letters seen 6 months after starting treatment. However, with time, the gain diminished, to an overall decline of 0.6 letters at the final follow-up visit.
DISSECTING VISION LOSS OVER TIME IN TREATED NAMD PATIENTS
Several reasons may account for the decline in visual acuity over time. First, suboptimal dosing can result in diminished visual gains. In the AURA study, the number of yearly injections differed between participating countries. More visits and injections were correlated with more successful maintenance of visual acuity gains. Similarly, in the SEVEN-UP study, a mean of 6.8 injections had been received since the exit from the HORIZON study (mean of 3.4 years). However, in patients who had received ≥11 injections, mean gain in letter score since exiting HORIZON was significantly better. This is partially a result of the difference in the treatment setting between well-controlled clinical trials and real life.
Given the advanced age of this treated population, frequent treatment is associated with significant burden for both patients and caregivers.17 Another explanation for the difference between clinical trials and real-life settings is the lack of incentive to perform more frequent injections. While such incentives exist in private practice settings, the public sector is less incentivized. Additionally, health care systems in some countries impose constraints on the number of permitted injections.10
Nevertheless, even newer treatment regimens designed to reduce the risk of suboptimal treatment, such as T&E, which is the favored treatment regimen in Australia, have not shown consistent maintenance of visual gains seen in years 1 and 2. Although VA is an important metric representing function, the macular morphology can reveal reasons for improved or indeed reduced visual function. In the SEVEN-UP study, the factor correlating most strongly with poor visual outcome was the increased area of macular atrophy.14 In the IVAN trial,18 less than one-third of eyes developed marked macular atrophy by the end of year 2, defined as atrophy within the footprint of the neovascular AMD lesion. These eyes had a numerically worse BCVA and near VA compared to eyes that did not have intralesional macular atrophy. Although these findings did not reach statistical significance, concerns remain regarding the potential for worse vision in eyes that develop macular atrophy.
In the long-term follow up of participants in the CATT study, the proportion of patients with an abnormally thin retina rose from 22% at the end of year 2 to 36% at 5 years. The study also found an increase in the prevalence of geographic atrophy (20% at 2 years and 41% at 5 years) and a substantial increase in lesion size (an increase of >50% over 3.5 years).11 In the SEVEN-UP study, fibrotic scars were demonstrated in one-third of study eyes, and nearly all eyes showed the presence of macular atrophy. The factor corresponding most strongly with poor visual outcome was increased area of macular atrophy while subretinal fibrosis did not have a significant effect on visual outcome.14
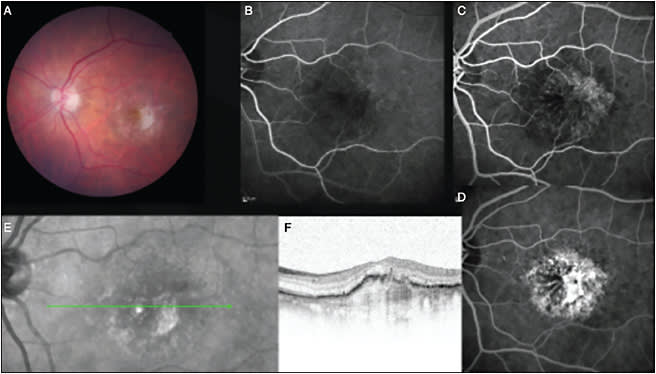
These data offer insight into the pathologic processes that continue even in the environment of lesion quiescence induced by anti-VEGF treatment. The underlying pathologic processes that define AMD continue to operate, resulting in both cellular loss and cellular disorganization. These processes result in worsening macular function even though the macula is maintained in a fluid-free environment (Figure 1). A further difference between populations enrolled in the randomized clinical trials and patients treated in routine practice is that patients with significant structural alterations in the macular retina are excluded from clinical trials. The irreversible nature of the structural damage to the macular retina at commencement of anti-VEGF therapy limits any functional improvement and likely has the potential to lead to continuing vision loss as the pathologic changes progress over time despite optimal treatment (Figure 2).

FUTURE DEVELOPMENTS IN NAMD
Although we appear to have reached a ceiling for vision gain and vision maintenance with anti-VEGF therapy, the recognition that atrophy and fibrosis are key factors in limiting both the gain and continuing benefit of treatment has led to a new wave of explorations to minimize these morphologic effects. Combination treatments to reduce fibrosis have been tried but have yielded disappointing findings.19,20 Currently, treatments are being explored to overcome the atrophic processes that are part of the AMD spectrum, and should any of these ongoing trials21 prove to be effective, the next logical step would be to combine drugs that prevent atrophy with the anti-VEGF agents. Phase 1 and 2 cell-replacement therapy trials in AMD are also ongoing to replace sick or defective RPE.22 Although AMD therapeutics are currently at a standstill, the future holds much promise as the various options for limiting or preventing fibrosis and atrophy of the macular retina are extensively researched.
In summary, we are better placed today compared to just over a decade ago, with an arsenal of anti-VEGF drugs that can ameliorate the VA loss due to untreated nAMD, and these play an important role in maintaining vision. Just more than one-third of people with nAMD when treated with anti-VEGF drugs continue to maintain VA of Snellen 20/40 or better, a goal which seemed unachievable in the past. Nonetheless, other therapeutic strategies are needed to ensure that a much higher proportion of patients will enjoy similar functional outcomes even in the longer term. RP
REFERENCES
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
- Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e5.
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548.
- Dakin HA, Wordsworth S, Rogers CA, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open. 2014;4(7):e005094-e005094.
- Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148(1):43-58.e1.
- Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398.
- Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-1267.
- Wykoff CC, Croft DE, Brown DM, et al; TREX-AMD Study Group. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122(12):2514-2522.
- Ba J, Peng R-S, Xu D, et al. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des Devel Ther. 2015;9:5397-5405.
- Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-226.
- Maguire MG, Martin DF, Ying G, et al; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Ophthalmology. 2016;123(8):1751-1761.
- Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175-1183.
- Doris N, Hart PM, Chakravarthy U, et al. Relation between macular morphology and visual function in patients with choroidal neovascularisation of age related macular degeneration. Br J Ophthalmol. 2001;85(2):184-188.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON. Ophthalmology. 2013;120(11):2292-2299.
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: Report 1: Visual acuity. Ophthalmology. 2014;121(5):1092-1101.
- Gillies MC, Campain A, Barthelmes D, et al; Fight Retinal Blindness Study Group.. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837-1845.
- Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361.
- Bailey C, Scott LJ, Rogers CA, et al; writing committee for the IVAN Study Group. Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology. 2019;126(1):75-86.
- Ophthotech announces results from third phase 3 trial of Fovista in wet age-related macular degeneration. https://www.businesswire.com/news/home/20170814005286/en/Ophthotech-Announces-Results-Phase-3-Trial-Fovista%C2%AE . Published August 14, 2017. Accessed February 5, 2019.
- Regeneron’s Eylea combination therapy fails mid-stage study. Reuters. https://www.reuters.com/article/us-regeneron-pharms-study-idUSKCN1201HW . Published September 30, 2016. Accessed February 5, 2019.
- Apellis Pharmaceuticals - Our Focus. http://www.apellis.com/focus-pipeline.html . Accessed February 7, 2019.
- Home. Gyroscope. http://gyroscopetx.com/ . Accessed February 7, 2019.








