For years, panretinal laser photocoagulation (PRP) remained the primary treatment modality for proliferative diabetic retinopathy (PDR), working by downregulation of vascular endothelial growth factor (VEGF) expression. However, the advent of anti-VEGF therapy has changed the treatment paradigm for PDR, as these agents are easy to administer and can treat neovascularization, as well as diabetic macular edema (DME). Surgical interventions, namely pars plana vitrectomy (PPV) with or without membrane peeling, play an instrumental role in the management of progressive manifestations of PDR, such as nonclearing vitreous hemorrhages (VH), vitreomacular traction, tractional retinal detachments (TRDs), combined TRD/rhegmatogenous retinal detachments (RRDs), and certain cases of DME.1,2 In this article, we aim to review the pathophysiology of PDR and offer a review of the medical and surgical treatment modalities available to retinal specialists to treat this condition and to improve patients’ visual potential.
PATHOPHYSIOLOGY
The pathophysiology of PDR stems from the microvascular changes associated with diabetes mellitus (DM). Progressive microvascular changes first lead to retinal hypoxia and later production of hypoxia-inducible factor, which stimulates a cascade of angiogenic growth factors including insulin-like growth factor 1, basic fibroblast growth factor, erythropoietin, VEGF, and other cytokines, such as IL-6, IL-8, and MCP-1.3,4 The vitreous cavity acts as a physiologic reservoir for these angiogenic growth factors, along with reactive oxygen species and advanced glycation end-products. These factors stimulate the development of neovascular buds on nearby retinal blood vessels and also cause thickening of the inner limiting membrane, often seen in DME, by affecting the footplates of Müller cells.4,5 Immature neovascular buds continue to proliferate and form firm adhesions to the vitreous, using the vitreous cortex as a scaffold for growth. Subsequent glial cell proliferation, fibrous tissue progression, and vitreous traction can cause recurrent VH due to the friable nature of the vessels, and further fibrous proliferation can ultimately cause formation of TRDs both in the periphery and in the posterior pole (Figure 1).
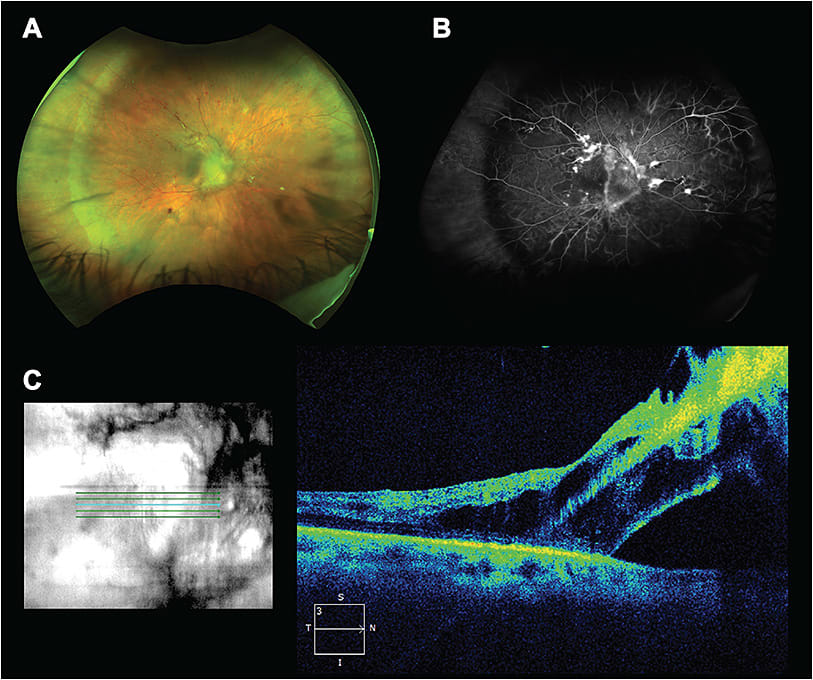
MEDICAL TREATMENT OPTIONS
A number of risk factors have been associated with the development of DR, including the total duration of DM, chronic hyperglycemia, hypertension, and ethnicity.2 Perhaps the most important consideration in the prevention of diabetic retinopathy (DR) is the effective control of modifiable risk factors. Studies such as the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications Study at 30 years (EDIC), have shown that intensive glucose control can reduce early microvascular disease secondary to type 1 diabetes mellitus (T1DM), including retinopathy, as well as later, more severe complications including cardiovascular disease.6 Tight glycemic control, with target preprandial blood glucose concentrations between 70 and 120 mg/dL, has proved effective at reducing the risk of developing DR with long-term benefits extending multiple decades.7 The vast majority of patients with DM have type 2 diabetes mellitus (T2DM), rather than T1DM; in these patients, tight glycemic control is also effective at reducing the need for PPV or PRP, as well as the development or progression of PDR.8
After the development of PDR, PRP is often a first-line treatment. Photocoagulation in the peripheral retina aims to reduce hypoxic growth factor production and induce regression of neovascular tissue.2 Although PRP does not accelerate clearance of VH, it does help to prevent progression to TRD by removing the vitreous scaffold, thereby significantly reducing the risk of long-term visual loss.4
However, since the FDA approval of anti-VEGF agents for DME and DR in the presence of DME, these agents have shown promising results for their application for PDR, as anti-VEGF agents are often used as an adjuvant to laser and surgical therapies. Anti-VEGF therapy, for example, results in rapid regression of retinal neovascularization.9 In Protocol S, the Diabetic Retinopathy Clinical Research Network compared the use of PRP to ranibizumab (Lucentis, Genentech) for PDR. This study found ranibizumab therapy to be noninferior to PRP treatment.10 Notably however, the ranibizumab group had a statistically significant advantage in visual acuity (VA) gain at 2 years, the PRP group experienced more peripheral visual field loss and underwent more vitrectomies (15% in the PRP group vs. 4% in the ranibizumab group), and ranibizumab was more effective in preserving central and peripheral vision when DME was also present. The one caution was that similar levels of patient compliance with follow-up may be difficult to achieve outside of a clinical trial setting. As such, for reliable patients presenting with PDR who are capable of frequent follow-up, anti-VEGF therapy may be a reasonable first-line treatment. Combination of anti-VEGF agents with PRP has proved effective at treating VH secondary to neovascularization and has reduced the need for PPV.11
Since intraoperative bleeding during PPV can obscure visualization and lead to increased surgical time and increased surgical failure, preoperative intravitreal bevacizumab (Avastin; Genentech) is often administered in attempt to reduce the overall surgery time, rate of intraoperative complications, and incidence of postoperative VH.12 In addition, administration of anti-VEGF therapy at 5 to 10 days prior to surgery is superior to more immediate 1- to 3-day preoperative timepoints, as evidenced by improved best-corrected VA and reduced postoperative complications in studies.13 Intravitreal injection of bevacizumab has been associated with the development of TRD, which is thought to be related to the proliferation of fibrous tissue or contraction of pre-existing fibrovascular membranes. As such, care should be taken when considering intravitreal bevacizumab in patients who have taut, active fibrovascular tissue present adjacent to the macula.14
Finally, Danis and colleagues found intravitreal dexamethasone implantation (Ozurdex; Allegan) to be an effective treatment modality for patients with DME, as it has shown to help delay the progression of diabetic retinopathy severity and reduce central subfield retinal thickness.15 Although the retreatment interval was ≥6 months in this study, a more frequent interval may be ideal, particularly for control of central subfield retinal thickness. When medical management fails, and more severe manifestations of PDR occur, PPV should be a consideration.
SURGICAL TREATMENT OPTIONS
PPV remains an instrumental surgical approach in the treatment of various stages of PDR. As previously discussed, the vitreous cavity can house a number of proinflammatory and angiogenic factors that induce changes resulting in neovascularization and DME. PPV thus can provide a reduction of these cytokines while also releasing the posterior hyaloid and dissecting the tractional fibrotic membranes that may cause a TRD.5 Although PPV for PDR was initially indicated only for nonclearing VH,16 the indications now also include extensive TRD, combined TRD/RRD, florid fibrovascular proliferations, vitreomacular traction, and reoperations for anterior hyaloidal proliferation in previously vitrectomized eyes.13,16 Outcomes have been steadily improving due in part to improvements in surgical technique, early diagnosis of diabetic manifestations, and increased willingness to operate on eyes with good VA (Figure 2).17
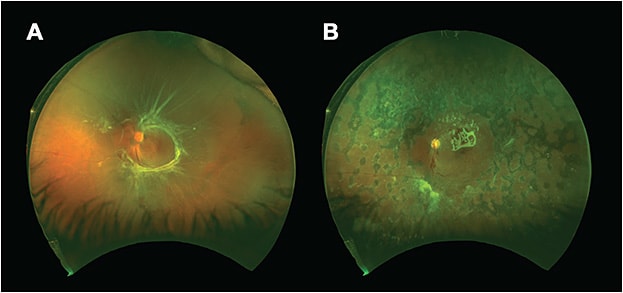
Timing of PPV is a critical consideration for retinal surgeons. For patients with VH and absence of an RD, as confirmed with ultrasonography, early PPV has proved useful for those with T1DM. For those with T2DM, PPV is helpful in the majority of patients if there has been no improvement after 4 weeks of observation.4 The Diabetic Retinopathy Vitrectomy Study found improvements in VA when PPV was completed within 6 months of severe VH.2 However, it should be noted that these findings are from a time before endolaser PRP was available and during the age of primarily 20-gauge vitrectomy. With smaller-gauge platforms and the availability of endolaser to perform PRP during PPV, current outcomes may be more favorable.
When a significant cataract limits the visualization of the posterior segment, a combined cataract extraction with phacoemulsification and intraocular lens insertion with PPV can also be performed with no increase in complications.16 Although PPV does greatly increase the rate of cataract progression in phakic patients, this rate after PPV for PDR is lower than after PPV for other indications, even after controlling for patient age, presence of gas tamponade, surgical time, or the presence of supplemental glucose in the vitrectomy infusion fluid.18 Since this difference is especially true in younger patients, one may consider postponing cataract surgery until a visually significant cataract develops. Furthermore, aphakic or pseudophakic patients with PDR are more prone to neovascularization of the iris and to neovascular glaucoma, often prompting delay in cataract surgery, if possible.4
PPV SURGICAL TECHNIQUE
The most common surgical approach for a PPV in DR is using a 3-port transconjunctival, sutureless, 23-gauge vitrectomy system. More recently, PPVs have been performed with smaller vitrectomy probes, particularly 25- and 27-gauge probes. Smaller-gauge vitrectomy systems are rising in popularity due to more controlled dissection of membranes with minimal underlying retinal tissue movement, less postoperative inflammation, and the ability to create smaller, self-healing sclerostomy wounds.19,20 Berrocal recently introduced the all-probe lift and shave technique, which uses either the smaller gauge 27-gauge or the 25-gauge vitrectomy probe, to engage fibrous tissue, lift with aspiration, and ultimately cut in a sequential fashion, with intermittent blunt dissection as needed. This technique allows for the shaving of tissue from the surface of the retina without the use of chandeliers, bimanual techniques, or scissors, with excellent anatomic reattachment and visual improvement.20
There are several approaches to initiating diabetic PPV. Typically a posterior vitreous detachment is not present. One technique involves completing a core vitrectomy with transection of the posterior hyaloid peripherally and relief of all anterior posterior traction prior to addressing more posterior pathology. This process can help to avert iatrogenic peripheral retinal tears during later dissection. Alternatively, one can initiate core vitrectomy with induction of a posterior vitreous detachment using the cutter or forceps at the optic nerve, although hemostasis may be an issue in the presence of neovascularization of the disc.
When addressing fibrotic tractional membranes, dissection can be performed in several ways. Early methods included en bloc dissection, whereby tractional membranes and the adherent posterior hyaloid are excised and removed as a single unit.21 More commonly, fibrovascular membranes are now dissected using viscodissection, segmentation, and/or delamination. Ancillary instrumentation to assist with membrane dissection can include retinal picks, vertical, horizontal, or mechanized scissors, tissue manipulators, spatulas, scrapers, and/or diamond knives, depending on surgeon preference.20 As mentioned previously, utilizing new high-cut rate vitreous cutters has enabled surgeons to often complete TRD repair using the cutter alone.
Fibrovascular membranes are typically dissected first off the optic nerve, where attachments are strongest, and then in the periphery to minimize iatrogenic retinal breaks. The goal should be to relieve as much traction as possible, especially in the area of the macula. Once membrane dissection is deemed satisfactory, a complete PRP can be performed to the ora serrata with scleral depression to reduce retinal hypoxic drive and minimize further neovascularization. Three hundred sixty-degree scleral depression is performed to identify any retinal breaks, and if any are noted, laser barricade is applied. The decisions of whether to place a tamponade agent and which agent to choose are extremely surgeon dependent and situational. In cases with no retinal breaks and completely relieved traction, leaving the eye with a fluid fill may be permissible. If tamponade is chosen, the options include air, sulfur hexafluoride (SF6) gas, perfluoropropane (C3F8) gas, and silicone oil. Gas is placed when possible, as silicone oil tamponade may aggravate postoperative preretinal membrane formation. However, if there are multiple large breaks, concerns about postoperative patient positioning, travel considerations, or the need for more immediate visual function (eg, a monocular patient) silicone oil can be employed for long-term tamponade (Figure 3).23
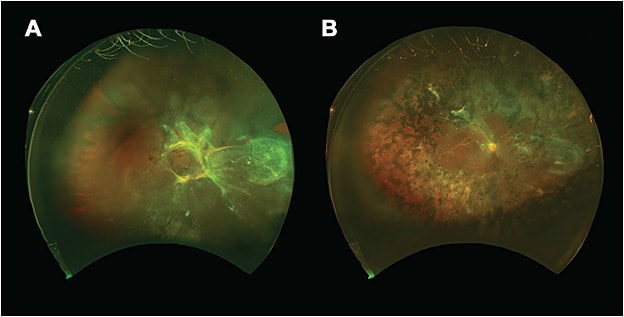
A bimanual technique has also been described for TRDs with good visual outcomes and rates of reattachment.16 With this technique, a self-retaining endoilluminator, referred to as a chandelier, is utilized, while both hands are used for careful dissection of membranes under direct visualization of the retina. Downsides of this technique, however, include the risk of phototoxicity if the lighted instrument is held too close to the retina, along with increased glare and difficulty placing the endoilluminator through smaller-gauge vitrectomy ports.
POSTOPERATIVE COMPLICATIONS
The most common complications of PPV for PDR include intraoperative and postoperative VH, less commonly with 27-gauge or 25-gauge systems vs a 23-gauge system.16 According to Motoda and colleagues, the most important predictor of postoperative VH is the duration of surgery, with mean durations of 71.5 min in patients with postoperative VH and 50.0 min in those without bleeding.24 Other possible predictors include fasting blood glucose immediately prior to surgery, history of treatment with antihypertensive drugs, and no history of treatment with antiplatelet drugs.24 The risk of postoperative VH can be minimized with thorough removal of peripheral vitreous gel, intraocular tamponade with perfluorocarbon, and preoperative intravitreal bevacizumab.4
Other complications of PPV include iatrogenic retinal breaks, especially during fibrovascular membrane dissection, sclerostomy-associated complications (such as tissue incarceration and fibrovascular ingrowth), and neovascular glaucoma.20 As mentioned previously, the risk of neovascular glaucoma is increased in pseudophakic or aphakic patients; treatment includes PRP and anti-VEGF therapy to reduce ischemia-induced neovascularization.4
PATIENT OUTCOMES
Anatomic and VA outcomes after PPV for PDR are generally favorable, with studies demonstrating significant VA improvements in 75% of eyes with TRDs25 and in 87% of eyes with diabetic VH.17 However, functional improvements are minimal when compared to focal/grid photocoagulation,2 which is why PPV is usually reserved for progressive complications of PDR. However, PPV techniques continue to improve with the addition of smaller-gauge instrumentation, and more recent studies have placed anatomic success rates at 98.1% and functional success rates at 80.6%, which are very promising for patients.
FOLLOW-UP
Postoperatively after PPV, patients are monitored closely to evaluate for further DR complications, such as recurrent neovascularization, VH or DME, retinal redetachments, proliferative vitreoretinopathy, and cataract and epiretinal membrane (ERM) formation. Reoperations are pursued when there is recurrent nonclearing VH or new traction. Visually significant cataracts and ERMs can be surgically removed to improve visual outcomes. Silicone oil tamponade can often be kept in the eye unless long-term complications arise, such as keratopathy, cataract formation, elevated intraocular pressure, or redetachment.26 For those patients who develop an ERM, estimated at 8% in a study by Shroff and colleagues,16 a membrane peel could be performed at the time of silicone oil removal.
CONCLUSION
In summary, PDR is an important vision-threatening complication in patients with DM. Although modifiable risk factor control is paramount for prevention of the disease, treatment is multimodal with a combination of both medical and surgical management approaches when progressive disease occurs. The mainstay of treatment of PDR involves PRP to reduce hypoxic drive and anti-VEGF therapy to control neovascularization and DME, but PPV is an important consideration for the treatment of later-stage complications of PDR. As surgical techniques and instrumentation continue to develop, patient outcomes are bound to improve, and surgeons will become more willing to operate on eyes with otherwise good VA. RP
REFERENCES
- Diabetic Retinopathy Clinical Research Network Writing Committee; Haller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087-1093.
- Vaziri K, Schwartz SG, Relhan N, Kishor KS, Flynn HW Jr. New therapeutic approaches in diabetic retinopathy. Rev Diabet Stud. 2015;12:196-210.
- Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol. 2013;Suppl 1(11).
- El Annan J, Carvounis PE. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int Ophthalmol Clin. 2014;54:141-153.
- Crim N, Velez-Montoya R, Morales-Canton V. Surgical versus medical treatment for diabetic macular edema: a review. Med Hypothesis Discov Innov Ophthalmol. 2017;6:136-142.
- Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9-16.
- Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
- Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695 e1-15.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137-2146.
- Huang YH, Yeh PT, Chen MS, Yang CH, Yang CM. Intravitreal bevacizumab and panretinal photocoagulation for proliferative diabetic retinopathy associated with vitreous hemorrhage. Retina. 2009;29:1134-1140.
- Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy a systematic review and meta-analysis. Retina. 2015;35:1931-1942.
- Castillo J, Aleman I, Rush SW, Rush RB. Preoperative bevacizumab administration in proliferative diabetic retinopathy patients undergoing aitrectomy: a randomized and controlled trial comparing interval variation. Am J Ophthalmol. 2017;183:1-10.
- Tranos P, Gemenetzi M, Papandroudis A, Chrisafis C, Papadakos D. Progression of diabetic tractional retinal detachment following single injection of intravitreal Avastin. Eye. 2008;22:862.
- Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100:796-801.
- Shroff CM, Gupta C, Shroff D, Atri N, Gupta P, Dutta R. Bimanual microincision vitreous surgery for severe proliferative diabetic retinopathy: outcome in more than 300 eyes. Retina. 2018 Feb 5. [Epub ahead of print]
- Gupta B, Sivaprasad S, Wong R, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye (Lond). 2012;26:510-516.
- Smiddy WE, Feuer W. Incidence of cataract extraction after diabetic vitrectomy. Retina. 2004;24:574-581.
- Anastasilakis K, Mourgela A, Kaniouras D, Moschou K. Tips, tricks, and advantages of 27-G vitrectomy. Ophthalmologica. 2018;239:176-177.
- Berrocal MH. All-probe vitrectomy dissection techniques for diabetic tractional retinal detachments: lift and shave. Retina. 2017 Oct 11. [Epub ahead of print]
- Abrams GW, Williams GA. “En bloc” excision of diabetic membranes. Am J Ophthalmol. 1987;103:302-308.
- Charles S. Vitrectomy techniques for complex retinal detachments. Taiwan J Ophthalmol. 2012;2:81-84.
- Adyanthaya R, Piwonka K. Tractional retinal detachment repair techniques. Retina Today. 2015 May/Jun:35-40.
- Motoda S, Shiraki N, Ishihara T, et al. Predictors of postoperative bleeding after vitrectomy for vitreous hemorrhage in patients with diabetic retinopathy. J Diabetes Invest. 2018;9:940-945.
- Tao Y, Jiang YR, Li XX, Gao L, Jonas JB. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina. 2010;30:447-451.
- Falkner C, Binder S, Kruger A. Outcome after silicone oil removal. Br J Ophthalmol. 2001;85:1324-1327.








