In 2017, medical history was made when voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics) received FDA approval and became the first gene therapy targeting a disease caused by specific gene mutations to be approved in the United States. The implications of this are truly profound, as a once-untreatable class of diseases suddenly became treatable. Reaching this point reflects a culmination of research efforts spanning decades and unlocks an array of promising possibilities in the field of inherited retinal diseases.
OVERVIEW
Voretigene neparvovec-rzyl is an adeno-associated virus vector (AAV)-based subretinal gene therapy indicated for patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy and viable retinal cells as determined by the treating physician(s). Biallelic mutations of the RPE65 gene disrupt the visual cycle and lead to accumulation of toxic byproducts in the retinal pigment epithelial (RPE) cells. RPE65 mutations cause 8-16% of Leber congenital amaurosis diagnoses and 1-3% of retinitis pigmentosa diagnoses.1-4 Symptoms and age of onset are variable, but affected patients generally complain of nyctalopia, peripheral field loss, and blurry vision, all of which usually progress over time. Subretinal injection of Luxturna induces transduction of some RPE cells with a cDNA encoding normal RPE65 protein.
CLINICAL STUDIES
Phase 1 studies of voretigene neparvovec-rzyl commenced in 2007, 10 years after RPE65 mutations were first identified. Given the favorable safety profile in 12 subjects, the highest tested drug dose (1.5 x 1011 vector genomes [vg] in 0.3 mL) was selected for use in future trials. The Phase 3 study (NCT00999609) completed enrollment of its 31 subjects ≥4 years of age in 2013. Control group subjects had the option to cross over to receive treatment after the first year post-enrollment, and all elected to proceed in this trial. Following pars plana vitrectomy, the drug was delivered as a 0.3 mL subretinal injection along the superior vascular arcade using a microtip cannula. Air-fluid exchange was performed with the intention to remove circulating vectors and tamponade the injection site. The second eye was injected 6 to 18 days later. The primary endpoint was functional vision measured by the Multi-Luminance Mobility Test (MLMT), which uses a mobility course to test subjects at light levels ranging from 1-400 lux. Other measured outcomes included full-field light sensitivity threshold (FST), which measures lowest illumination detected over the entire retina, visual acuity (VA), and visual field.
At year 1, the mean bilateral MLMT change score was 1.8 (SD 1.1) light levels in the treatment arm versus 0.2 (SD 1.0) in the control group (difference of 1.6, 95% CI 0.72-2.41, p=0.0013), and there was a 100-fold FST improvement in treated patients. Thirteen (65%) of 20 treated subjects, but no control participants, passed the MLMT at the lowest luminance level. VA improvements in those treated were modest, but not statistically significant. No serious adverse events related to the product or its administration, such as a clinically significant cytotoxic T-cell response to AAV2 or RPE65, were observed at 1 year.5 These positive effects have been sustained for at least 3 years, as recently presented by Stephen Russell, MD, professor of ophthalmology at University of Iowa, at the 2018 Annual Meeting of the American Society of Retina Specialists.6
POST-FDA APPROVAL
Following FDA approval, Spark Therapeutics proceeded with a staged product rollout. There are currently nine treatment centers administering Luxturna: Baylor College of Medicine, University of Iowa, Massachusetts Eye & Ear Infirmary, Bascom Palmer Eye Institute, Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital, Casey Eye Institute, Children’s Hospital Los Angeles, and Scheie Eye Institute. All participating surgeons have completed the Spark Therapeutics Surgical Education Program. Given its first-in-class status as an ophthalmic gene therapy, several important precedents have been set in terms of pricing (the drug is priced at $425,000 per eye), distribution (Express Scripts has partnered with Spark Therapeutics for distribution and specialty pharmacy services, circumventing a buy-and-bill model), and payer agreements (outcomes-based pricing is being practiced in certain plans).
PRE-OPERATIVE PLANNING
Careful planning is critical to ensure treatment success. When a potential candidate is identified, genetic testing must be performed to confirm that a biallelic RPE65 mutation is present; ancillary tests, which may include visual fields, perimetry, electroretinogram, optical coherence tomography, autofluorescence, FST, and fundus photography, may guide assessment of a patient’s visual potential. If the decision is made to move forward, detailed patient counseling is imperative to discuss expectations, risks, and benefits of treatment. Additionally, the benefits investigation should be initiated at this point. Spark Therapeutics offers a program called Generation Patient Services to assist in this process as well as to navigate reimbursement, coverage, travel logistics, and out-of-pocket costs.
Once a surgery date has been set, it is important to disseminate logistical information to all involved parties. Aaron Nagiel, MD, PhD, assistant professor of ophthalmology at University of Southern California/Children’s Hospital Los Angeles, best describes the process: “[It’s] truly a team effort. A diligent clinical coordinator, adept pharmacists, and experienced OR staff are just a few examples of essential personnel that facilitate a smooth surgical delivery.”
Our facility found it extremely beneficial to rehearse day-of-surgery events 1 to 2 weeks prior to the actual operation, especially because our institutional pharmacy is geographically distanced from the surgical center, which adds one additional drug transfer point. A practice run-through also allows the pharmacy staff to review the drug preparation process and provides the surgical team an opportunity to handle the supplies.
SURGICAL DELIVERY OF LUXTURNA
With regard to Luxturna delivery, there are numerous variations in surgical approach and technique. Therefore, although a few approaches are discussed in this section, this does not imply that one is more effective than others.
Luxturna should be administered in an aseptic environment. Two prepared syringes (one for back-up) with 0.8 mL of diluted drug each should be provided by pharmacy personnel. A 41-gauge subretinal injection cannula with a polyamide microtip and a polyvinyl chloride extension tube with an inner diameter of ≤1.4 mm and length of ≤15.2 cm are also needed in addition to the normal set-up for a pars plana vitrectomy.7
Many surgeons prefer general anesthesia for their patients either because of pediatric age or to minimize movement. The eye should be prepared and draped as per normal sterile protocol. Connect the first syringe to the extension tube and subretinal injection cannula (Figure 1). Prime the apparatus by injecting until droplets are seen at the cannula tip; some elect to then continue injecting until the plunger tip aligns with the 0.3 mL mark so that the intended delivery volume is set; others prefer to bypass this step in the event that additional drug is needed.
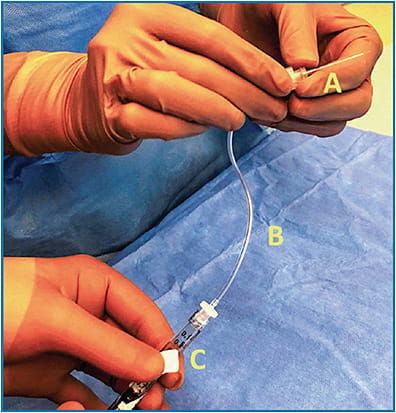
A standard 3-port pars plana vitrectomy should be performed using any gauge of choice. Most agree that the posterior hyaloid should be elevated; avoid triamcinolone acetonide stain, if possible.
“Interestingly, the vitreoretinal interface in these patients tends to make the hyaloid quite easy to detach, even in younger children,” says Audina Berrocal, MD, professor of ophthalmology at Bascom Palmer Eye Institute.
Once complete, the intended site of administration should be identified. Ideally, this is located along the superotemporal arcade, at least 2 mm distal to the foveal center, and not in direct contact with retinal vessels or areas with pathologic features. Some surgeons will cut a bevel at the cannula tip to facilitate entry into the subretinal space. Regarding its insertion through the port, “We found it helpful to remove the valve of the superotemporal cannula with 0.12 forceps to prevent kinking of the subretinal cannula,” advises Tahira Scholle, MD, assistant professor of ophthalmology at Baylor College of Medicine.
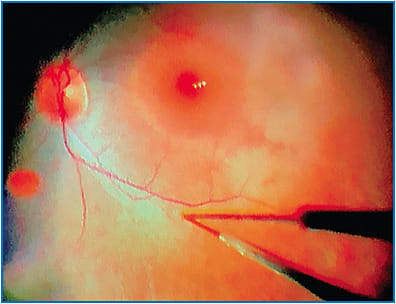
While the surgical assistant is holding the syringe, the surgeon will bury the cannula tip into the intended injection site (Figure 2) and tell the assistant to begin slowly injecting until a subretinal bleb is visualized. Recently, some surgeons have utilized a foot pedal-assisted method to inject the vector. A total volume of 0.3 mL (1.5 x 1011 vg) should be delivered (Figure 3). In cases where it is difficult to raise a subretinal bleb, some have used balanced salt solution or even air to initiate a bleb and then subsequently injected the drug into it; how this affects drug concentration or localization is not fully understood at this time.
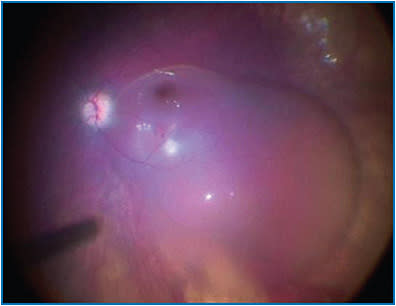
After completing the injection, retract the subretinal cannula from the eye and discard all unused product per local biosafety guidelines. Perform an air-fluid exchange, avoiding drainage near the retinotomy site. Proceed with closure as per normal protocol. Supine head positioning should be initiated immediately in the post-operative period and maintained as much as possible over the first 24 hours. Typical post-operative surveillance and drop regimen are followed in addition to a short course of oral corticosteroids (typically started 3 days prior to Luxturna administration). The second eye should be treated no sooner than 6 days after the first eye.
CONCLUSION
It is an exciting time for all retina specialists, especially for those in the early stages of their careers. Luxturna is not only the first FDA-approved targeted gene therapy, but also represents a giant leap forward for all patients with inherited retinal diseases. As our knowledge of genetic disorders and innovative gene therapies continues to advance, it is fathomable that we will be able to treat other gene mutation-associated conditions in the future. NRP
REFERENCES
- Thompson DA, Gyürüs P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41(13)4293-4299.
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci USA. 1998;95(6):3088-3093.
- Fahim AT, Daiger SP, Weleber RG. Nonsyndromic Retinitis Pigmentosa Overview. In: Adam MP, Ardinger HH, Pagon RA et al., eds. GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2018. Aug 2, 2000 [updated Jan 19, 2017].
- Stone EM. Leber congenital amaurosis — a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144(6):791-811.
- Russell S, Bennett J, Wellman J, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomized, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-860.
- Russell S. 3-Year Update for the Phase 3 Voretigene Neparvovec-rzyl Study in Biallelic RPE65 Mutation–Associated Inherited Retinal Disease. Presented at: American Society of Retina Specialists 36th Annual Meeting; July 2018; Vancouver, BC, Canada.
- Spark Therapeutics. Highlights of Prescribing Information. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM589541.pdf ; Last accessed July 31, 2018.









