The diabetic retina patient population is associated with higher incidences of serious disease manifestation and rapid disease progression, compared to the nondiabetic population.1 Accordingly, these patients often create a significant management burden for the practices treating them, requiring more consultations and therapy - both surgical and pharmacological - than their peers.
As retina specialists, we are always looking for more effective ways to diagnose, treat, and manage these patients while reducing operational strain on our practices. Recent advances in optical coherence tomography (OCT) imaging technology help us diagnose and manage diabetic patients with greater speed and clarity, affording us the opportunity to provide superior quality care and to increase efficiency in the lane.
BENEFITS IN A HIGH-VOLUME PRACTICE
Victor Gonzalez, MD
My retina practice spans multiple locations in Texas’s Rio Grande Valley. Due to local demographics, I see several hundred diabetic patients every month, many of whom present with advanced retinal disease, including significant retinopathy. Many of these patients have had diabetes for years before their initial presentation, and disease progression is often rapid.
Unlike other patients, whom we may choose to follow up once per year, these patients require frequent visits and increased time and attention. Patients who present with diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) must be managed monthly. Research implies that, at this stage, diligent, strategic care is critical, potentially leading to reduced vision loss over the long term.2
To ensure that we can process as many patients as possible while providing top-quality care, we leverage every tool at our disposal, including imaging technology, to improve efficiency when possible. In the case of OCT, newer swept-source technology offers several benefits that increase our efficiency.
Unlike spectral-domain OCT (SD-OCT), swept-source-OCT (SS-OCT) is capable of rapidly visualizing a larger area in greater detail.3 Its superior speed, scope, and image quality allow us to visualize the vitreous, retinal structure, and choroid in one take. As a busy practitioner, this ability is very helpful, as it allows me to diagnose and manage with greater nuance. For instance, if I discover subretinal fluid as part of my work-up, I can quickly determine whether the fluid is the result of retinopathy or a choroidal lesion. In the past, I would have had to move the patient to an ultrasound machine to provide a complete image, increasing our time burden and causing some patient discomfort. Since implementing SS-OCT, we have reduced patient wait times and time in the chair. Our device of choice (DRI OCT Triton; Topcon) captures approximately 100,000 images in seconds, creating a high-resolution image in a short amount of time. Swept-source OCT as a technology offers multiple clinical advantages. First, it provides a high-quality image of the retina and choroid, even in the presence of challenging media, such as cataracts, blood, and gas. The former factor is especially relevant given the high comorbidity of cataract in the diabetic patient population.3 Imaging these patients with SD-OCT would require ultrasound imaging, which - while sometimes necessary - can be an additional burden for patient and practice alike.
The clinical and efficiency advantages provided by SS-OCT make it useful in any practice seeing a large volume of diabetic retina patients. Having every important diagnostic test required for in-depth evaluation of a diabetic patient in 1 device, in 1 room, creates an environment of high speed, high efficiency, and high-quality care, empowering our practitioners and creating lasting benefits for patients.
OVERCOMING DIAGNOSTIC CHALLENGES
Netan Choudhry, MD, FSCRC
Diabetic patients are frequent guests in our Toronto-based practice. In the past, imaging and diagnosing these patients using SD-OCT presented several challenges. Patients with opaque media in the eye, such as cataracts and hemorrhages, were difficult to image with clarity and speed. Additionally, having fewer imaging modalities in a single device meant we needed more machines, a larger footprint in the office, and a longer time to process all our retinal patients, those with diabetes included.
Since implementing SS-OCT in our practice, we have improved efficiency and clinical efficacy by imaging every retina patient using our device. In fact, my technicians often vie for use of our SS-OCT device, as it simplifies and speeds up almost every aspect of a patient’s diagnostic workup. Its speed and image quality provide value in every patient case, but I find it especially beneficial in cases in which serious complications, such as preretinal hemorrhages and macular holes, are present.4
I recently published a study to assess the utility of SS-OCT in visualizing macular hole closure through gas-filled eyes on postoperative day 1, when we determined that SS-OCT with dual-scanning protocol and manual delicate focusing enabled consistent early visualization of foveal architecture for assessment of hole closure in gas-filled eyes.5 This ability is just one unique advantage that SS-OCT technology may offer.
Another area in which I achieve excellent clinical value using SS-OCT is in patients with peripheral retinal disease (Figure 1). Historically, imaging retina patients with peripheral disease was difficult and produced suboptimal results, largely due to patient discomfort and, subsequently, movement during imaging. Newer SS-OCT technologies scan rapidly and use a subvisible 1,050-nm wavelength, improving patient comfort and reducing the likelihood that a patient will move and disrupt the imaging process.

Ultimately, SS-OCT helps us to produce an image of very high quality in almost any situation in which it would not have been possible using older technology, and this change is highly relevant in the diabetic population, in which serious disease, rapid progression, and complicating factors are prevalent.
Additionally, integration with our practice’s electronic medical record system is useful and appreciated. When we image a patient with our SS-OCT device — which is capable of capturing fundus photography, in addition to SS-OCT and OCT angiography (OCTA) — the patient’s images are uploaded automatically to his or her digital health record, saving time. Integration with the EMR was easy to set up, but your mileage may vary depending on your device and software or cloud-based EMR vendors.
SWEPT-SOURCE OCTA FOR DIABETIC PATIENTS
Roger A. Goldberg, MD, MBA
I have been fortunate over the past few years to collaborate with Carl Zeiss Meditec on the development of next-generation retinal imaging technology, including both hardware and software upgrades to SD-OCT, SS-OCT, and OCTA. On the AngioPlex, Zeiss’s SD-OCTA platform, 68,000 A-scans can be acquired per second. This forces a trade-off between resolution and the size of the scan pattern.7 Therefore, when using SD-OCTA, I ask my technicians to acquire 3 mm x 3 mm scan patterns for choroidal diseases, because I want the best resolution to look for choroidal neovascular membranes above or below the retinal pigment epithelium. When using the AngioPlex for vascular diseases like retinal vein occlusion or diabetic retinopathy, I ask my technicians to use the 6 mm x 6 mm or 8 mm x 8 mm scan patterns, because I am primarily looking for retinal nonperfusion or flow in retinal neovascular tissue.
In contrast, when using the Zeiss Plex Elite SS-OCTA, the trade-off between resolution and scan size is largely eliminated. This device currently scans at 100,000 A-scans per second, and a modified next generation device is able to acquire 200,000 A-scans per second. Coupled with the real-time eye tracking to eliminate motion artifacts, the resolution is excellent, even with “widefield” scans of 12 mm x 12 mm, or 15 mm x 9 mm scans, which can be auto-montaged to create 15 mm x 15 mm OCTA en-face images (Figure 2). Therefore, I can standardize the scan pattern across my patients; this makes the job easier for my technicians and photographers.
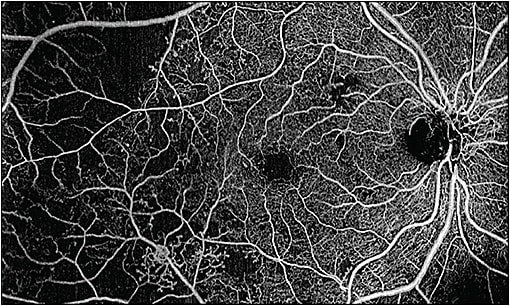
The complete view of the vascular flow throughout the posterior pole is helpful in diabetic patients to look for flow not only in the retina, but above the retina as well in what Zeiss calls the VRI slab (vitreo-retinal interface; above the internal limiting membrane). With the high resolution, widefield OCTA provided by a swept-source device, I can get great detail of the foveal avascular zone, such as the size and shape, as well as peripheral nonperfusion in a single scan. Similarly, because of the oversampling and image-averaging in each individual B-scan, my technicians do not need to acquire both a structural OCT scan and an OCTA scan; the quality in each B-scan is excellent, and the flow overlay on each B-scan can be added or removed depending on what I am looking for.
In addition, the VRI slab can be used to look for flow (and changes in flow) in neovascular tissue. We know from decades of experience that sometimes patients with treated PDR bleed from what clinically appears to be fibrotic, “inactive” neovascularization. Using OCTA to image the flow in this neovascular tissue, and treating with anti-VEGF injections when flow increases on OCTA, may reduce the incidence of recurrent vitreous hemorrhages in these patients. Widefield SS-OCTA makes following these patients easy over time.
THE TAKEAWAY
There are now several companies offering SS-OCTA both in the United States and abroad. SS-OCT offers the advantage of faster scan speed, which translates clinically into better resolution with wider fields of view. For diabetic patients with both macular and peripheral pathology, this is clinically useful, especially as patients may require repeated imaging over time. In addition, this technological improvement confers benefits to busy clinical practices as well, by enabling faster, more efficient throughput, and standardization of imaging protocols. RP
REFERENCES
- Van Leiden HA, Dekker JM, Moll AC, et al. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121(2):245-251.
- Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol. 2016;172:72-79.
- Pham TQ, Wang JJ, Rochtchina E, Maloof A, Mitchell P. Systemic and ocular comorbidity of cataract surgical patients in a western Sydney public hospital. Clin Exp Ophthalmol. 2004;32(4):383-387.
- Choudhry N, Golding J, Rao RC. Seeing through walls: subhyaloid hemorrhage. Ophthalmology. 2016;123(6):1172.
- Li DQ, Choudhry N. Swept-source OCT visualization of macular hole closure in gas-filled eyes. Ophthalmic Surg Lasers Imaging Retina. 2017;48(5):392-398.
- Choudhry N, Golding J, Rao RC. Widefield swept-source OCT of retinal capillary hemangioblastoma. Ophthalmol Retina. 2017;1(4):321.
- Goldberg RA. Practical tips for incorporating OCT angiography into your practice. Retinal Physician. 2017;14(7):30-32. Available at https://www.retinalphysician.com/issues/2017/september-2017/practical-tips-for-incorporating-oct-angiography-i .








