Pigment epithelial detachment (PED) in neovascular age-related macular degeneration (nAMD) is a pathologic finding where the retinal pigment epithelium (RPE) separates from the underlying Bruch’s membrane due to the accumulation of fluid, fibrovascular membrane, blood, or drusenoid material.1 Classification of the various forms of PEDs is based on appearance on clinical exam, spectral domain optical coherence tomography (SD-OCT), and fluorescein angiography (FA).2-4 Fibrovascular PEDs are commonly found in nAMD patients and are notoriously difficult to treat with the current generation of anti-VEGF pharmacologic agents.5-8 Up to 66.5% of eyes with nAMD are noted to have PEDs,9 and the majority of clinical trials have either excluded these eyes or have not performed the necessary subanalyses to evaluate the response of PED to treatment. PEDs have previously been associated with poor visual prognosis as they are often unresponsive or develop tolerance to pharmacotherapies over time.10-12
PATHOPHYSIOLOGY
The mechanism responsible for fibrovascular PED formation within nAMD is not well understood. PED formation appears directly related to vascular growth (such as choroidal neovascularization [CNV]) through Bruch’s membrane that leaks fluid (either serous or hemorrhagic) into the potential space between Bruch’s membrane and the RPE. This fluid accumulation elevates the surrounding hydrostatic pressure and detaches the RPE from the underlying Bruch’s membrane, resulting in the clinical findings of a PED.1
What factors are responsible for the therapeutic resistance of PEDs and its persistence within the nAMD eye over time? This is an active area of research, and recent developments may provide an answer. A potential mechanism may be related to the abundance of lipid-rich extracellular deposition within the Bruch’s membrane found in normal aging.13,14 These lipid deposits can manifest as soft drusen as well as basal linear deposits, which are hallmark findings of AMD. Over a period of decades, the lipid deposition may create a hydrophobic barrier to decrease outward passage of fluid through Bruch’s membrane.14-16 Therefore, once fluid accumulates within the potential space between the RPE and Bruch’s membrane to form PEDs, it is very difficult to remove, thus explaining their resistance to treatment.
DIAGNOSIS
Clinically, a PED appears as a smooth dome-shaped subretinal elevation on fundoscopy and may be associated with other nAMD findings such as drusen, subretinal fluid, hemorrhage, or overlying RPE hyperplasia. Fibrovascular PED in type 1 CNV appears as an irregular RPE elevation with or without serous exudate. A flattened or notched border of the PED may hint at an associated CNV membrane.1 Evaluating a PED with conventional SD-OCT demonstrates a smooth or irregular elevation of the retinal pigment epithelium with associated sub-RPE fluid (serous), vitelliform material, or blood. SD-OCT may also reveal the CNV membrane appearing as a hyperreflective material on the posterior surface of the lesion. On FA, sub-RPE stippled hyperfluorescence in the early phase will slowly increase to produce staining of the sub-RPE.17 Serous PED in nAMD is caused by leakage from CNV. SD-OCT shows an elevation of RPE overlying a homogenous hyporeflective space, while the FA reveals intense early hyperfluorescence and progressive rapid pooling within the PED. Indocyanine green angiography (ICGA) may further help to differentiate serous and vascularized PEDs, as serous is typically hypofluorescent while focal CNV shows early or late staining.17

Nevertheless, the microvascular complex of the causative CNV in a fibrovascular PED can be difficult to visualize using conventional angiography or SD-OCT. OCT angiography (OCTA) is a novel noninvasive imaging modality that may provide more clarity. This methodology evaluates the retinal and choroidal microvasculature using amplitude decorrelation and phase-variance to detect vascular flow without using intravascular dyes. En face structural images and cross-sectional B scans with blood flow signals are viewed along with a 3D microvasculature map. OCTA can be used to distinguish vascular PEDs from non-neovascular PEDs, assisting in disease diagnosis.18 Non-neovascular PEDs, including drusenoid and serous PEDs, have no flow signal within or adjacent to the lesion and appear as dark areas on the en face image. Vascular PEDs show a tangle of neovascular network with flow signal usually with a distinguishable feeder vessel on the cross-sectional scan. OCTA additionally has the potential to identify subclinical neovascularization in nonexudative lesions, which may provide prognostic information.19

TREATMENT: DO WE NEED TO FLATTEN PEDS?
Anti-VEGF Therapy
Anti-VEGF pharmacotherapies such as bevacizumab (Avastin; Genentech), ranibizumab (Lucentis; Genentech), and aflibercept (Eylea; Regeneron) have become the mainstay of treatment for nAMD. Despite their proven success in resolving subretinal and intraretinal fluid associated with CNV, their ability to resolve PEDs appears to be limited. No clear consensus exists as to an effective treatment protocol for PEDs in the setting of nAMD, and, historically, the presence of PEDs is not a recommended parameter in dictating retreatment in a treat-and-observe algorithm.
In general, studies using all 3 anti-VEGF therapeutics have demonstrated a reduction of PED lesion size and stabilization of visual acuity.20,21 Analyses from the VIEW and CATT trials both demonstrated slow and incomplete resolution of PED, in contrast to intraretinal cysts and subretinal fluid, which responded to anti-VEGF therapy more robustly.22-24 Visual gains have been correlated with resolution of intraretinal and subretinal fluid, not with resolution of the PED.
In a post-hoc analysis of the HARBOR trial, a 24-month phase III randomized multicenter double-masked study25 that compared 2 doses of ranibizumab (0.5 mg vs 2.0 mg) given monthly or as-needed for treating nAMD, Sarraf et al showed that all treatment arms had similar best corrected visual acuity (BCVA) gains regardless of the presence or height of the PED at baseline. Although the 2.0 mg dosage group showed a slightly better anatomic response as well as a higher rate of PED resolution than the 0.5 mg dosage group, there was no significant difference in BCVA outcomes. In the as-needed treatment group, there was a trend toward more treatments given as PED height increased. Although the presence of a PED was a retreatment criterion in the HARBOR trial, patients with complete resolution of PED did not necessarily see an additional benefit in BCVA and were more likely to demonstrate macular atrophy after 24 months of treatment. Furthermore, the PED subtype was not assessed. Conversely, another prospective study of 36 eyes comparing 0.5 mg vs 2.0 mg doses of ranibizumab given monthly or as-needed for vascular PEDs demonstrated similar visual acuity and morphological outcomes, but the higher dosage resulted in earlier reductions and more complete resolution of the PED.26 However, there was also an increased tendency for the formation of an RPE tear with the higher dosage, especially for eyes with larger PED dimensions. A post-hoc study thereafter showed no differences between fibrovascular PED vs serous PED.27 In the RECOVER trial, a prospective multicenter study, 40 patients with vascular PEDs received monthly ranibizumab treatment. There was a moderate increase in BCVA, and PED height and diameter decreased at 1 year in patients with serous PEDs without RPE tear. By contrast, in fibrovascular PEDs, BCVA as well as anatomical findings remained stable. One-quarter of patients with serous PEDs developed an RPE tear with a subsequent decrease in BCVA.28

Recent studies have suggested that switching therapeutic agents from bevacizumab or ranibizumab to aflibercept may confer a better functional and anatomic outcome in refractory cases of nAMD. Small prospective studies that investigated patients with persistent vascular PED despite bevacizumab or ranibizumab who switched to aflibercept using 3 loading doses followed by an as-needed regimen showed an anatomical improvement; however, in most studies there were no significant BCVA changes.29-32 The exception was a recent larger multicenter, prospective, nonrandomized trial of treatment-resistant PEDs, which showed an improvement of 3.3 Early Treatment of Diabetic Retinopathy Study letters after 8 months after switching from as-needed ranibizumab to a fixed aflibercept regimen.33
In a post-hoc analysis of the VIEW2 trial, a 96-week phase 3 randomized, double-masked, multicenter trial of aflibercept vs ranibizumab, Schmidt-Erfurth et al argued that PED is the most relevant parameter reflecting disease progression.34 Following 3 loading doses of anti-VEGF therapy, PEDs resolved in less than half of affected eyes, whereas intraretinal cyst and subretinal fluid resolved in 70% to 80% of eyes. All anatomic changes reached a plateau after the third month for both scheduled and as-needed treatment regimens. Intraretinal cysts had the lowest BCVA compared to subretinal fluid or PED. Interestingly, this study also observed that the occurrence of intraretinal cysts associated with loss of BVCA was distinctly restricted to when the regimen was switched from scheduled to as-needed in patients with PED. They hypothesize that PED recurrence is a primary event in neovascular reactivation that leads to secondary neurosensory cystic degeneration, which itself results in the greatest functional loss for patients. As such, the formation of PED may underlie the therapeutic disadvantage of as-needed treatment regimen compared to scheduled treatments.
Photodynamic Therapy With Verteporfin
Laser therapies including direct thermal laser photocoagulation of PED, ICG-guided photocoagulation of CNV feeder vessels, and PDT have been attempted with minimal visual benefit.35-41 Large clinical trials investigating laser therapy for nAMD such as TAP, MPS, and VIP have excluded patients with serous PEDs. Our current understanding is limited to smaller retrospective studies that have shown mixed results.38,42,43 Small pilot studies combining PDT with anti-VEGF therapy suggest this approach may show some anatomical and functional gains over either treatment alone.44-47
COMPLICATION OF THERAPY: RPE TEARS
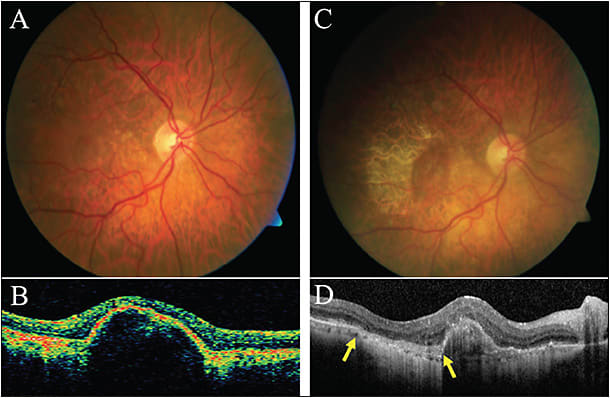
RPE tear or rip is a complication that may cause significant sudden visual loss, especially in subfoveal lesions. It may develop both spontaneously in vascularized PEDs as well as during the course of anti-VEGF treatment. In the natural course of PED, the subretinal fluid applies hydrostatic pressure to stretch the monolayer of RPE. Contraction of the CNV membrane adherent to the undersurface of the RPE increases the tangential tractional forces, creating an RPE separation at the edge of the PED opposite to the CNV. Anti-VEGF therapy causes an additional increase on contraction of the CNV membrane, increasing the risk for RPE tear. Clinical exam shows a well-defined hyperpigmented line representing rolled-up RPE adjacent to an area of hypopigmented bare choroid, which can be best detected on fundus autofluorescence (FAF). OCT shows hyper-reflectivity of the retracted RPE and an interruption of the RPE monolayer. FA shows a subtle ring of hyperfluorescent window defect at the margin of the PED.
The spontaneous rate of RPE tear in the natural history of PED within nAMD has been reported to be between 3% and 12.5%.3,4,48,49 With anti-VEGF therapy, reported incidence in patients with vascular PEDs vary significantly between studies, ranging from 2.8% to 24% after ranibizumab treatments.36,50-52 Most RPE tears occur during the 3 loading doses of anti-VEGF therapy. The most recognized risk factor for RPE tear is a large PED height, with the cut-off ranging from 400 μm to 600 μm among different studies.53-57 Additional risk factors include larger PED diameters and a small ratio of CNV size to PED size.50,57,58 OCT findings such as RPE thinning, wavy RPE indentations, irregular contractile RPE folds, and small holes or interruptions along the PED margin may be predictive of impending tears.59-61 Radial hyper-reflective lines in near-infrared reflectance imaging may also be a predictive finding.58
High-risk patients with vascular PEDs may benefit from more frequent examination with SD-OCT, FAF, and near-infrared imaging; however, evidence for preventing an RPE tear is still lacking. While some have proposed discontinuing anti-VEGF therapy with frequent follow-up in high-risk patients until these predictors decline,62 anti-VEGF therapy is still needed to treat subretinal fluid and prevent fibrosis and formation of an end-stage exudative disciform scar in order to maintain visual acuity.53,63-66 Others favor ranibizumab or bevacizumab to aflibercept for these patients because of a theoretical increased potency for CNV membrane contraction with aflibercept.67 The relative safety of these agents in respect to the risk of RPE rips, even among big/tall PEDs has not been established. A biweekly half-dose of anti-VEGF therapy has been proposed to reduce the risk for RPE tears; however, this remains controversial.68,69 Earlier studies comparing 0.5 mg vs 2.0 mg of ranibizumab suggested that the higher dose resulted in increased risk of RPE tears,26,55 but in a more recent study, the rates were found to be comparable.25
CONCLUSIONS
PEDs remain a predictor of poor visual outcome in nAMD. While anti-VEGF therapies, in either monthly or as-needed treatment regimens, have been shown to decrease the size of the PED, visual improvements have mainly been attributed to the resolution of intraretinal and subretinal fluid instead of reducing the PED. Our experience has been that any newly vascularized PED that is not associated with intraretinal or subretinal fluid and with preserved visual acuity can be safely observed with frequent follow-up. Consequently, flattening of PEDs should not be a primary or secondary treatment goal as it is not necessary or can even be detrimental (ie, worsening atrophy) in eyes with PEDs and neovascular AMD. When treating PEDs, we must be wary of RPE tears, which are associated with significant vision deterioration. The mechanisms driving PED formation and its persistence remain an active area of research. With improved understanding, we may further refine our treatment algorithms, whether with existing anti-VEGF agents or novel therapies, to optimize visual outcomes for our patients. RP
REFERENCES
- Gass JD. Serous retinal pigment epithelial detachment with a notch. A sign of occult choroidal neovascularization. Retina. 1984;4(4):205-220.
- Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984;68(8):513-519.
- Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985;69(6):397-403.
- Pauleikhoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002;240(7):533-538.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444.
- CATT-Research-Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201.
- Coscas F, Coscas G, Souied E, Tick S, Soubrane G. Optical coherence tomography identification of occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2007;144(4):592-599.
- Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96(1):1-2.
- Poliner LS, Olk RJ, burgess D, Gordon ME. Natural history of retinal pigment epithelial detachments in age-related macular degeneration. Ophthalmology. 1986;93(5):543-551.
- Singerman LJ, Stockfish JH. Natural history of subfoveal pigment epithelial detachments associated with subfoveal or unidentifiable choroidal neovascularization complicating age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1989;227(6):501-507.
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95(12):1638-1645.
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42(1):265-274.
- Bird AC. Bruch’s membrane change with age. Br J Ophthalmol. 1992;76(3):166-168.
- Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch’s membrane. Invest Ophthalmol Vis Sci. 1995;36(7):1290-1297.
- Mrejen S, Sarraf D, Mukkamala SK, Freund KB. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina. 2013;33(9):1735-1762.
- Tan ACS, Freund KB, Balaratnasingam C, Simhaee D, Yannuzzi LA. Imaging of pigment epithelial detachments with optical coherence tomography angiography. Retina. 2018;38(9):1759-1769.
- Kang H, Byeon SH, Kim SS, Koh HJ, Lee SC, Kim M. Combining en face optical coherence tomography angiography with structural optical coherence tomography and blood flow analysis for detecting choroidal neovascular complexes in pigment epithelial detachments. Retina. 2018 May 8. [Epub ahead of print]
- Inoue M, Arakawa A, Yamane S, Kadonosono K. Variable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy. Retina. 2013;33(5):990-997.
- Panos GD, Gatzioufas Z, Petropoulos IK, Dardabounis D, Thumann G, Hafezi F. Effect of ranibizumab on serous and vascular pigment epithelial detachments associated with exudative age-related macular degeneration. Drug Des Devel Ther. 2013;7:565-569.
- Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(7):3115-3120.
- Jaffe GJ, Martin DF, Toth CA, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatment trials. Ophthalmology. 2013;120(9):1860-1870.
- Sharma S, Toth CA, Daniel E, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(4):865-875.
- Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology. 2016;123(10):2213-2224.
- Chan CK, Abraham P, Sarraf D, Nuthi AS, Lin SG, McCannel CA. Earlier therapeutic effects associated with high dose (2.0 mg) Ranibizumab for treatment of vascularized pigment epithelial detachments in age-related macular degeneration. Eye (Lond). 2015;29(1):80-87.
- Chan CK, Sarraf D, Abraham P. Treatment outcomes of conventional or high-dose ranibizumab for vascularized pigment epithelial detachment based on lesion subtypes. Eur J Ophthalmol. 2018. [Epub ahead of print]
- Clemens CR, Wolf A, Alten F, Milojcic C, Heiduschka P, Eter N. Response of vascular pigment epithelium detachment due to age-related macular degeneration to monthly treatment with ranibizumab: the prospective, multicentre RECOVER study. Acta Ophthalmol. 2017;95(7):683-689.
- Broadhead GK, Hong T, Zhu M, et al. Response of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degeneration. Retina. 2015;35(5):975-981.
- Kim K, Kim ES, Kim Y, Yang JH, Yu SY, Kwak HW. Outcome of intravitreal aflibercept for refractory pigment epithelial detachment with or without subretinal fluid and secondary to age-related macular degeneration. Retina. 2017. [Epub ahead of print]
- Major-Jr JC, Wykoff CC, Croft DE, et al. Aflibercept for pigment epithelial detachment for previously treated neovascular age-related macular degeneration. Can J Ophthalmol. 2015;50(5):373-377.
- Tyagi P, Juma Z, Hor YK, Scott NW, lonean A, Santiago C. Clinical response of pigment epithelial detachment associated with neovascular age-related macular degeneration in switching treatment from Ranibizumab to Aflibercept. BMC Ophthalmol. 2018;18(1):148.
- Blanco-Garavito R, Jung C, Uzzan J, et al. Aflibercept after ranibizumab intravitreal injections in exudative age-related macular degeneration: the ARI2 study. Retina. 2017 Dec 21. [Epub ahead of print]
- Schmidt-Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122(4):822-832.
- Costa RA, Rocha KM, Calucci D, Cardillo JA, Farah ME. Neovascular ingrowth site photothrombosis in choroidal neovascularization associated with retinal pigment epithelial detachment. Graefes Arch Clin Exp Ophthalmol. 2003;241(3):245-250.
- Lommatzsch A, Heimes B, Gutfleisch M, Spital G, Zeimer M, Pauleikhoff D. Serous pigment epithelial detachment in age-related macular degeneration: comparison of different treatments. Eye (Lond). 2009;23(12):2163-2168.
- Pece A, Isola V, Vadalá M, Calori G. Photodynamic therapy with verteporfin for choroidal neovascularization associated with retinal pigment epithelial detachment in age-related macular degeneration. Retina. 2007;27(3):342-348.
- Axer-Siegel R, Ehrlich R, Rosenblatt I, et al. Photodynamic therapy for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Arch Ophthalmol. 2004;122(4):453-459.
- Wygnanski-Jaffe T, Desatnik H, Alhalel A, et al. ICG angiography-guided photodynamic therapy for large pigment epithelial detachments in age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006;37(5):358-363.
- Gomez-Ulla F, Gonzalez F, Abelenda D, Rodriguez-Cid MJ. Diode laser photocoagulation of choroidal neovascularization associated with retinal pigment epithelial detachment. Acta Ophthalmol Scand. 2001;79(1):39-44.
- Lim JI, Aaberg TM, Capone Jr A, Sternberg Jr P. Indocyanine green angiography-guided photocoagulation of choroidal neovascularization associated with retinal pigment epithelial detachment. Am J Ophthalmol. 1997;123(4):524-532.
- Coscas F, Stanescu D, Coscas G, Soubrane G. Feeder vessel treatment of choroidal neovascularization in age-related macular degeneration. J Fr Ophtalmol. 2003;26(6):602-608.
- Introini U, Torres-Gimeno A, Scotti F, Setaccioli M, Giatsidis S, Bandello F. Vascularized retinal pigment epithelial detachment in age-related macular degeneration: treatment and RPE tear incidence. Graefes Arch Clin Exp Ophthalmol. 2012;250(9):1283-1292.
- Ladas ID, Kotsolis AI, Papakostas TD, Rouvas AA, Karagiannis DA, Vergados I. Intravitreal bevacizumab combined with photodynamic therapy for the treatment of occult choroidal neovascularization associated with serous pigment epithelium detachment in age-related macular degeneration. Retina. 2007;27(7):891-896.
- Maier M, Haas K, Feucht N, et al. Photodynamic therapy with verteporfin combined with intravitreal injection of bevacizumab for occult and classic CNV in AMD. Klin Monbl Augenheilkd. 2008;225(7):653-659.
- Shima C, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Tano Y. One-year results of combined photodynamic therapy and intravitreal bevacizumab injection for retinal pigment epithelial detachment secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):899-906.
- Gonzalez A, Khurshid G. Treatment of retinal pigment epithelial detachment secondary to exudative age-related macular degeneration. Am J Ophthalmol Case Rep. 2017;9:18-22.
- Hartnett ME, Weiter JJ, Garsd A, Jalkh AE. Classification of retinal pigment epithelial detachments associated with drusen. Graefes Arch Clin Exp Ophthalmol. 1992;230(1):11-19.
- Chuang EL, Bird AC. Repair after tears of the retinal pigment epithelium. Eye (Lond). 1988;2(Pt 1):106-113.
- Chiang A, Chang LK, Yu F, Sarraf D. Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina. 2008;28(9):1265-1269.
- Guber J, Praveen A, Saeed MU. Higher incidence of retinal pigment epithelium tears after ranibizumab in neovascular age-related macular degeneration with increasing pigment epithelium detachment height. Br J Ophthalmol. 2013;97(11):1486-1487.
- Smith BT, Kraus CL, Apte RS. Retinal pigment epithelial tears in ranibizumab-treated eyes. Retina. 2009;29(3):335-339.
- Doguizi S, Ozdek S. Pigment epithelial tears associated with anti-VEGF therapy: incidence, long-term visual outcome, and relationship with pigment epithelial detachment in age-related macular degeneration. Retina. 2014;34(6):1156-1162.
- Leitritz M, Gelisken F, Inhoffen W, Voelker M, Ziemssen F. Can the risk of retinal pigment epithelium tears after bevacizumab treatment be predicted? An optical coherence tomography study. Eye (Lond). 2008;22(12):1504-1507.
- Sarraf D, Chan C, Rahimy E, Abraham P. Prospective evaluation of the incidence and risk factors for the development of RPE tears after high- and low-dose ranibizumab therapy. Retina. 2013;33(8):1551-1557.
- Chan CK, Abraham P, Meyer CH, et al. Optical coherence tomography-measured pigment epithelial detachment height as a predictor for retinal pigment epithelial tears associated with intravitreal bevacizumab injections. Retina. 2010;30(2):203-211.
- Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007;27(5):541-551.
- Clemens CR, Bastian N, Alten F, Milojcic C, Heiduschka P, Eter N. Prediction of retinal pigment epithelial tear in serous vascularized pigment epithelium detachment. Acta Ophthalmol. 2014;92(1):e50-e56.
- Shiraki K, Kohno T, Ataka S, Abe K, Inoue K, Miki T. Thinning and small holes at an impending tear of a retinal pigment epithelial detachment. Graefes Arch Clin Exp Ophthalmol. 2001;239(6):430-436.
- Moroz I, Moisseiev J, Alhalel A. Optical coherence tomography predictors of retinal pigment epithelial tear following intravitreal bevacizumab injection. Ophthalmic Surg Lasers Imaging. 2009;40(6):570-575.
- Nagiel A, Freund KB, Spaide RF, Munch IC, Larsen M, Sarraf D. Mechanism of retinal pigment epithelium tear formation following intravitreal anti-vascular endothelial growth factor therapy revealed by spectral-domain optical coherence tomography. Am J Ophthalmol. 2013;156(5):981-988.
- Ersoz MG, Karacorlu M, Arf S, Sayman-Muslubas I, Hocaoglu M. Retinal pigment epithelium tears: classification, pathogenesis, predictors, and management. Surv Ophthalmol. 2017;62(4):493-505.
- Durkin SR, Farmer LD, Kulasekara S, Gilhotra J. Change in vision after retinal pigment epithelium tear following the use of anti-VEGF therapy for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016;254(1):1-6.
- Lesniak SP, Fine HF, Prenner JL, Roth DB. Long-term follow-up of spontaneous retinal pigment epithelium tears in age-related macular degeneration treated with anti-VEGF therapy. Eur J Ophthalmol. 2011;21(1):73-76.
- Moreira-Jr CA, Arana LA, Zago RJ. Long-term results of repeated anti-vascular endothelial growth factor therapy in eyes with retinal pigment epithelial tears. Retina. 2013;33(2):277-281.
- Sarraf D, Joseph A, Rahimy E. Retinal pigment epithelial tears in the era of intravitreal pharmacotherapy: risk factors, pathogenesis, prognosis and treatment (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112(142-159).
- Clemens CR, Eter N. Retinal pigment epithelium tears: risk factors, mechanism and therapeutic monitoring. Ophthalmologica. 2016;235(1):1-9.
- Monés J, Biarnés M, Badal J. Bimonthly half-dose ranibizumab in large pigment epithelial detachment and retinal angiomatous proliferation with high risk of retinal pigment epithelium tear: a case report. Clin Ophthalmol. 2013;7:1089-1092.
- Nagiel A, Sadda SR, Schwartz SD, Sarraf D. Resolution of a giant pigment epithelial detachment with half-dose aflibercept. Retin Cases Brief Rep. 2015;9(4):269-272.








