Multiple sclerosis (MS) is a disease resulting from demyelination and inflammation of the central nervous system (CNS). The cause is unknown; autoimmunity, neurodegeneration, and latent or persistent viral infection have all been proposed.1 It is the most common immune-related disorder of the CNS, with about 2.3 million people (incidence 2.5/100,000 per year and prevalence 30/100,000 population) affected worldwide.1,2 However, there are wide geographic variations in prevalence, ranging from less than 0.5/100,000 in Africa to 8.3/100,000 in the Americas to 80/100,00 in Europe (and >200/10,000 in some northern European populations.3 The age at onset ranges from childhood to the 60’s, but most patients become symptomatic in their third to fourth decade of life.1 Overall, women are affected about twice as often as men, although the ratio decreases in patients with older age of onset.4
Average life expectancy is about 30 years from the diagnosis of disease (about 5-10 years less than normal). About 18,000 deaths worldwide are attributed to MS yearly (including suicide and infections).5
There are four phenotypes of MS as described by the National Multiple Sclerosis Society and the Multiple Sclerosis International Federation:6,7
- Clinically isolated syndrome (CIS),
- Relapsing-remitting MS (RRMS),
- Primary progressive MS (PPMS), and
- Secondary progressive MS (SPMS).
These phenotypes are descriptive only and do not reflect differences in pathophysiology.7 Most patients with CIS will evolve to RRMS, the most common phenotype of MS. Some patients develop progressive onset MS from the onset of disease (PPMS) while others develop progressive disease only after a course of RRMS (SPMS). An excellent summary of multiple sclerosis is provided by Thompson et al.7
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
The 2001 McDonald criteria8 and subsequent revisions9 are most commonly used to diagnose and classify MS, using a combination of clinical, laboratory, electrophysiologic, and magnetic resonance imaging (MRI) findings. Based on the methods used, clinicians find a diagnosis of MS, possible MS (those at risk of MS but in whom the diagnosis is equivocal), or not MS. The objective evidence of lesions typical of MS that are separated over time and space is essential in the diagnosis and helps to exclude other differential diagnoses. Acceptable evidence of MS lesions found by MRI include at least 3 of the 4 following criteria: (1) 1 gadolinium-enhancing lesion or 9 T2 hyperintense lesions if enhancing lesions are not present, (2) at least 1 infratentorial lesion, (3) at least 1 juxtacortical lesion, and (4) at least 3 periventricular lesions.9 Controversy persists regarding the diagnostic accuracy of lesions found by MRI in the spinal cord in the absence of brain involvement. Most CNS lesions are relatively small, but tumefactive demyelinating lesions are rare (1-3/1,000 cases of MS), usually solitary, and greater than 2 cm. They can mimic neoplasms.10 While 2 clinical attacks of MS, clearly separated in time and space, may be sufficient to make a diagnosis of MS, the absence of MRI, CSF, or VEP abnormalities should make the diagnosis of MS suspicious.9
Supportive laboratory testing using cerebrospinal fluid (CSF) includes the presence of oligoclonal IgG bands not found in the serum and the presence of an elevated IgG index. Lymphocytic pleocytosis should be less than 50/mm.3 CSF analysis can be particularly helpful in atypical clinical presentations or when MRI testing has reduced specificity (eg, older patients).8,9 Visual evoked potential (VEP) testing classically shows delayed potential with a well-preserved wave form.8,9
The differential diagnosis of MS is broad and includes infection (HTLV-1, Lyme disease, syphilis, progressive multifocal leukoencephalopathy), monophasic demyelinating diseases (acute disseminated encephaloymelitis, neuromyelitis optica, and acute transverse myelitis), ischemic/inflammatory disorders (neurosarcoidosis, antiphospholipid antibody syndrome, Takayasu disease, disseminated lupus erythematosus, and CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukocencephaolpathy), toxins (alcohol, ethambutol), and genetic disorders of myelin (such as the leukodystrophies).8
TREATMENT OF MULTIPLE SCLEROSIS
There is no cure for MS. The most common therapy for acute attacks is intravenous corticosteroids, followed by a short course of oral steroids.5 As of 2018, a number of disease-modifying drugs for relapsing or primary progressive MS have been licensed (interferon beta-1a, interferon beta-1b, interferon beta-1a, glatiramer acetate, natalizumab, fingolimod, alemtuzumab, teriflunomide, mitoxantrone, dimethyl fumarate, peginterferon beta-1a, cladribine, and ocrelizumab).7 However, the significant advances in the treatment of RRMS have not been as beneficial for progressive MS. Only ocrelizumab has been shown to slow progression in patients with PPMS.7 Tumefactive demyelinating lesions are particularly difficult to treat.10 Several recent publications summarize the role of disease-modifying treatment in MS.11,12
Immunosuppressive therapy for the treatment of MS has numerous risks, and the potential adverse drug effects must always be weighed against potential benefit. Furthermore, while the life expectancy of patients with MS has increased significantly due to better drug therapy, clinicians must consider the impact of aging when considering treatment options.13 Awareness of drug-induced side effects is essential when adjunctive therapy is used to treat MS-related complications such as fatigue, impaired ambulation, bladder dysfunction, erectile dysfunction, constipation, depression, cognitive impairment, neuropathic and musculoskeletal pain, and non-uveitic ocular problems such as oscillopsia.7
Thompson et al7 strongly recommended comprehensive management approach in all patients with MS that enhances quality of life, promotes wellness, addresses aggravating factors, and manages comorbidities. Certainly, the management of MS-associated uveitis should follow the same guidelines.
MS-ASSOCIATED UVEITIS
Ocular involvement in MS most commonly includes optic neuritis14 and oculomotor palsies.15 However, uveitis is an important complication of MS as well. There is undoubtedly an association between MS and uveitis, but the reported prevalence and incidence varies considerably. Olsen and Frederiksen16 extensively reviewed the published literature on the epidemiology of MS-associated uveitis and noted the many difficulties in getting reproducible data. These include a change in the diagnostic criteria for MS over time, the type of uveitis assessed (all types, intermediate uveitis only, anterior uveitis only, or vasculitis only), the differences in the definition of different types of uveitis (the widely used Standardization of Uveitis Nomenclature [SUN] was not published until 2005),17 size of the populations studied, geographic differences, and general lack of prospective analyses. In smaller studies, the range for prevalence of MS in patients with uveitis was 0.7% to 30.4% and the prevalence of uveitis in patients with MS was 0.9% to 36.7%. However, in studies involving 1,000 patients or more, the prevalence of MS among patients with uveitis was 0.9% to 1.7%, and the prevalence of uveitis among patients with MS was 0.65% to 1.1% (well above the prevalence in the general population of 0.0196%18 to 0.115%).18,19 A German study of 1,916 patients with uveitis found MS in 59 (3.1%); however, among patients with intermediate uveitis only, MS was the cause in 46%.20
In their review of the 5 largest studies of MS and uveitis,21-25 Olsen and Frederiksen16 found no clear pattern as to whether uveitis or MS presents first. The age of onset at presentation was 26.5 years to 36.5 years for MS and 23.3 years to 37.2 for uveitis. This does not include a small retrospective study of 17 children with MS and 15 with probable MS, in which 1 patient had progressive uveitis.26 The onset between uveitis and MS is quite variable and the gap may be wide. Jordan et al27 also described an 8-year-old girl who had bilateral uveitis at the age of 8 and then developed MS at age 21.
Two large studies of patients with MS and uveitis in whom the specific type of uveitis (anterior, intermediate, posterior, or panuveitis) was analyzed showed that intermediate uveitis was the most common presentation, occurring in 61%23 to 80%24 of patients. In contrast, Biousse et al21 found a similar percentage of panuveitis (39.3%) and intermediate uveitis (35.7%) in patients with uveitis, and Le Scanff et al22 found that panuveitis was the most common type (35.7%), followed by anterior (28.6%) and panuveitis (28.6%). Among patients with intermediate uveitis, MS is the third most common cause, behind pars planitis and sarcoidosis.28 It is important to keep in mind, though, that the diagnostic criteria for different types of uveitis have changed over time. Prior to publication of the SUN criteria in 2005, many investigators conflated vitritis, mild anterior chamber cells, and retinal vasculitis (with or without macular edema) with panuveitis, whereas under the SUN criteria the same findings would be considered intermediate uveitis.17
Most studies of patients with MS and uveitis in which data were analyzed by gender showed a female predominance of between 60% and 100%, although a Danish study found that only 38% were women.29
CLINICAL FEATURES OF MS-ASSOCIATED UVEITIS
A wide range of clinical features of uveitis have been described in patients with multiple sclerosis.23,30,31,32 Eighty percent to 93% of patients have intermediate uveitis,30,31 but anterior uveitis is an under-recognized presentation, occurring in 15% of patients.23 Jouve et al37 described 36 patients with MS-associated uveitis, of whom the majority (69.4%) were female. The uveitis was usually bilateral (88.9%), granulomatous (61%), and more commonly recurrent (69.4%) than chronic (27.8%). Of 15 patients described by Hedayatfar et al,32 93% were female, and the inflammation was bilateral and chronic in all patients. Messenger et al23 noted that patients with MS-associated intermediate uveitis were more likely female and older (mean age 40.6 years) than those with idiopathic intermediate uveitis.
Other complications include glaucoma, posterior synechiae, cataract, epiretinal membrane, vitreous hemorrhage, retinal periphlebitis, retinal neovascularization, macular edema, subretinal fluid, and traction retinal detachment. Glaucoma and cataract, of course, may also be secondary to corticosteroid therapy in the treatment of uveitis. Figures 1-5 show examples of some of these sequelae of MS-associated uveitis. While macular edema in MS is usually secondary to uveitis, or occasionally to the use of fingolimod,33,34 Gelfand35 found that 15 of 318 (4.7%) patients with MS had microcystic retinal edema with no other risk factors, such as uveitis or retinal vascular occlusion. In addition, patients with macular edema had worse vision, worse disability scores, and higher MS severity scores than patients without macular edema. The edema was more common in patients with prior episodes of optic neuritis.


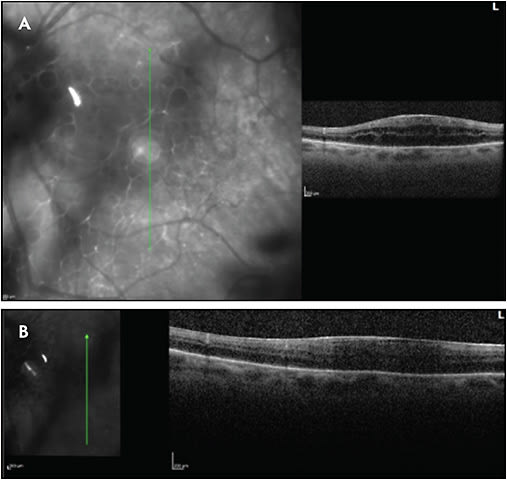


Periphlebitis occurs in more than half of patients with MS-associated uveitis.31,32 Sepulcre et al36 found in a study of 61 patients with MS that retinal periphlebitis was a slight risk factor for the development of relapse of MS within the next 2 years (odds ratio 1.52), and patients with retinal periphlebitis had larger gadolinium-enhancing lesions. However, several large studies found no major differences in the clinical features of MS in patients with or without uveitis.22,24,37 Similarly, most studies have found that the type of uveitis (intermediate uveitis vs nonintermediate uveitis) and the time of onset of uveitis (before or after the onset of MS) do not affect neurologic disability.21,22,37 However, a more recent study using newer diagnostic criteria for MS found that MS patients with uveitis had significantly lower neurologic disability scores; patients with uveitis were more than 200 times more likely to have benign MS than controls.24
WORKUP OF UVEITIS
In a patient with known MS and a clinical ocular examination consistent with MS-associated uveitis, no further workup is necessary. However, in patients with uveitis in whom MS is in the differential diagnosis but has not yet been identified, the workup should follow the same principles as with any other uveitis workup.38 A careful history, thorough eye examination, highly directed review of systems based on the clinical findings, and then a parsimonious collection of imaging (eg, fluorescein angiography, OCT), laboratory, and radiologic tests should be undertaken. The ophthalmologist can be helpful in both helping to confirm a diagnosis of MS and ruling out MS when the underlying systemic disease is in question. The differential diagnosis for granulomatous uveitis, intermediate uveitis, or uveitis with neurologic manifestations is broad and includes pars planitis, sarcoidosis, Vogt-Koyanagi-Harada disease, Behcet disease, primary vitreoretinal lymphoma, tuberculosis, toxoplasmosis, toxocariosis, syphilis, bartonella, and echinococcosis.
Many uveitis specialists will routinely order a small set of tests for virtually all patients with uveitis to help rule out sarcoidosis and syphilis, because these two diseases can cause almost any type of uveitis.38,39 Thus, a chest x-ray (or chest CT scan), serum angiotensin-converting enzyme level, and syphilis serology are commonly ordered. However, the use of a “uveitis panel” (a “one-size-fits-all” approach) in the workup of uveitis is to be discouraged, because it is not cost effective and is likely to yield a false-positive result. For example, it is useless to obtain HLA B27 testing in a patient presenting with bilateral intermediate uveitis, because such a presentation would not be explained by the presence of the gene, and a positive result would have a minimal positive predictive value.
Smith and Rosenbaum40 retrospectively reviewed the records of 1,450 patients over a 15-year period and found that 115 (7.9%) had neurologic disease causally related to the uveitis. Of the 115 patients, 14 (1% of all patients and 12.2% of patients with uveitis and neurologic symptoms) had MS. Pars planitis or bilateral granulomatous uveitis was found in three-quarters of affected patients. A combination of certain clinical features, positive neurologic review of systems, and patient age consistent with MS should therefore prompt the consideration of MS.
If immunosuppression is going to be considered for treatment of uveitis, it is important to rule out MS in the workup, because the anti-tumor necrosis factors drugs (such as adalimumab and infliximab) and the IL-6 inhibitor tocilizumab sometimes used to treat noninfectious uveitis may worsen demyelinating disease and are contraindicated in patients with MS.41 A complete blood count and comprehensive metabolic panel should be ordered to be sure there are no hematologic, renal, or hepatic contraindications to certain immunosuppressive drugs.41 Similarly, interferon gamma releasing assays are mandatory before initiating biologic anti-inflammatory therapy, because such drugs may worsen latent or active tuberculosis, HIV, hepatitis B, hepatitis C, and toxoplasmosis.41 Similarly, progressive multifocal leukoencephalopathy (PML), a potentially fatal disease caused by the polyomavirus JC, may develop in patients treated with immunosuppressive drugs such as natalizumab, rituximab, and mycophenolate mofetil.42,43 For reasons that are not clear, the risk of PML in patients with MS is much higher (as much as 1 in 1,000) when treated with natalizumab compared to other disease-modifying agents such as dimethyl fumarate, fingolimod, rituximab, ocrelizumab, and cladribine.42 Testing for antibodies to the JC virus is now routine prior to initiation of biologic therapy for MS, and at the very least, neurologic consultation should be obtained by ophthalmologists prior to initiating immunosuppressive therapy for MS-associated uveitis.
Raja et al44 retrospectively followed 37 patients with pars planitis and found that 6 patients (16.2%) developed MS. The odds ratio for patients developing MS was 2.86 in those patients carrying the HLA-DR15 allele. Nonetheless, in a review of HLA typing in the diagnosis of uveitis, Zamecki and Jabs45 noted the HLA typing does not distinguish between pars planitis and MS-associated intermediate uveitis, and therefore the HLA typing has marginal diagnostic value (and presumably even lower value in other forms of uveitis).
Should ophthalmologists routinely order MRI of the brain in patients with uveitis to “rule out” MS? The risk of converting from clinically isolated syndrome to clinically definite MS after 5 years in the Optic Neuritis Treatment Trial was 51% for patients with baseline brain MRI scans containing ≥3 lesions,46 and there is widespread agreement that patients with MS should undergo neurologic imaging as part of the workup for MS.
The correlation between uveitis and development of MS is much less clear. Prieto et al47 reported a prospective series of patients with intermediate uveitis and found that 43.5% had demyelinating lesions; RRMS was eventually diagnosed in 30.4% of patients. The authors’ findings supported the role of routine MRI in the workup of patients with intermediate uveitis. Raja et al44 found in a retrospective study that 11% of patients with intermediate uveitis had MS, and the risk was greater in those patients with retinal vascular sheathing. Jakob et al20 found that 10.3% of more than 400 patients with intermediate uveitis subsequently received a diagnosis of MS, and Gordon and Goldstein48 found the lifetime prevalence of MS to be 10-fold higher in patients with intermediate uveitis.
However, other studies have not replicated such a high incidence of MS in patients with intermediate uveitis. Furthermore, Prieto et al47 made no distinction in the workup between patients with and without neurologic symptoms; the diagnostic yield of MRI in patients with intermediate uveitis would presumably be much lower in the latter group. In each of these studies, the authors suggest that MRI should be performed in patients with uveitis.
Petrushkin et al39 argue that patients should not undergo routine MRI testing as part of the workup for intermediate uveitis. While there is evidence that early treatment of MS results in improved outcomes, Alshamrani et al49 and Okuda50 argue that patients with even just radiologically isolated syndrome (asymptomatic demyelinating lesions) should be treated with disease-modifying therapy. Petrushkin et al39 point out that studies have not found a difference in visual outcome in uveitis patients with demyelinating lesions and those without. Furthermore, only about 30% of patients with demyelinating lesions on MRI develop MS within 5 years,51 and Labiano-Fontcuberta and Benito-Leon52 recommend against treatment of radiologically isolated syndrome.
Clearly, there are inadequate data to answer the question about the role of neurologic imaging in patients with uveitis. Petrushkin et al39 recommend that the diagnosis of MS should be made by a neurologist (or neuro-ophthalmologist), because it is not made on the basis of MRI findings alone. Patients with symptoms of MS or those with high-risk characteristics of uveitis, such as intermediate uveitis with retinal vasculitis, should be referred promptly to a specialist. A neurologist is far better trained to order and interpret the proper test (for example, whether the spinal cord should be imaged), thus conserving costs and maximizing the yield of the workup.53
PATHOPHYSIOLOGY OF MS AND MS-ASSOCIATED UVEITIS
Multiple factors have been associated with the development of MS, including age, gender, genetics (presence of HLA DRB1*15:01 allele), nutritional factors (decreased vitamin D), geography (distance from the equator), and lifestyle (eg, cigarette smoking).7 At the cellular level, both the innate and adaptive immune systems are involved in the pathogenesis, mediated through microglia, activated macrophages, and both B and T lymphocytes.7
The cause of uveitis in patients with MS is unknown, although it may be an autoimmune reaction that results from sensitization of the immune system to antigens expressed in the central nervous system; myelin basic protein and myelin oligodendrocyte glycoprotein have been showed in animal models to promote autoimmune uveitis.54 Nerve tissue and ocular tissue derive from the same embryonic cells, so MS and uveitis may share some etiologic factors.16
Vascular cell adhesion molecule-1 (VCAM-1) is a ligand for alpha-4 integrin receptors, which promote lymphocyte action and inflammation.10 Adhesion molecules may also play a role in the development of uveitis, suggesting the potential of VCAM-1 inhibitors for treatment of both MS and uveitis.
TREATMENT OF MS-ASSOCIATED UVEITIS
Overall, the visual prognosis of patients with uveitis and MS is good compared to patients with idiopathic intermediate uveitis. Messenger et al23 found no statistically significant different difference between patients with MS and intermediate uveitis and those with idiopathic intermediate uveitis. The 2 fundamental questions about treatment of MS-associated uveitis are: (1) in a patient with uveitis and undiagnosed MS, does the treatment for uveitis have any effect on the course of MS? and (2) In a patient with both MS and uveitis, does the treatment of the MS affect the course of the uveitis?
There is no evidence that corticosteroid therapy for uveitis influences the risk of developing MS. Thus, patients with anterior or intermediate uveitis should be treated with topical or regional corticosteroid injections as in other cases of noninfectious uveitis. Immunosuppressive therapy was necessary in only 13.9% of patients in one study,31 and it should be considered for posterior or panuveitis and severe intermediate uveitis with the distinct exception of tumor necrosis factor inhibitors such as infliximab or adalimumab. Such drugs may potentiate demyelinating diseases;55 they are contraindicated in patients with MS and should be avoided if at all possible in patients with high-risk characteristics, such as intermediate uveitis and retinal periphlebitis.56,57
The effect of MS treatment on the course of uveitis has been addressed in several studies. Jouve et al31 retrospectively evaluated outcomes in 36 patients with RRMS and uveitis, of whom 19 patients were treated for MS and 17 were not at the time of diagnosis of uveitis. The mean age of onset of MS and uveitis was 35 and 32 years, respectively. Uveitis occurred before the onset of neurologic symptoms by a mean of 4.9 years in 28 patients and neurologic symptoms occurred before the onset of uveitis by a mean of 4.7 years in the other 8 patients. Isolated granulomatous uveitis was found in only 3 of 12 of patients. The most common type of uveitis was pars planitis (86%), and in this group of patients, retinal periphlebitis and snowballs were the most common findings. There was no significant difference in the ocular findings between the 2 groups. The authors found a good overall prognosis of uveitis and no significant difference in the rate of uveitis relapse between the treated and untreated groups.
In contrast, several articles have suggested that interferon beta may favorably affect the course of MS-associated uveitis. Becker et al58 studied the efficacy of interferon beta-1a in 13 patients, in which 71% of patients had improved vision and 69% were off steroids after 2 years of interferon therapy. Mackensen et al59 compared the efficacy of interferon beta-1a (IFN) and MTX for the treatment of intermediate uveitis with macular edema of multiple causes, including MS. The patients treated with interferon had significantly less macular thickening at 3 months compared with patients treated with methotrexate. However, visual acuity of these patients only slightly. Jouve et al31 pointed out that the study of Mackensen et al included patients with non-MS-related intermediate uveitis and theorized that patients with non-MS-related intermediate uveitis may have had a better response to interferon beta than patients with MS-related uveitis.
Roemer et al10 reported a case of a patient with MS, intermediate uveitis, retinal vasculitis, and tumefactive demyelinating lesion who had dramatic resolution of the ocular findings within 1 month of treatment. Becker et al examined interferon as a treatment for MS-associated uveitis.58 Seventy-one percent of patients had improved visual acuity, and 69% were off steroids after 2 years of therapy.
A small study by Hedayatfar et al32 showed that mycophenolate mofetil can be an effective corticosteroid-sparing treatment for MS-associated uveitis. Alemtuzumab, a humanized monoclonal antibody against cell surface CD52 that has been approved for treatment of MS,60 was shown in a single case report to control MS-associated uveitis and macular edema in a patient with concurrent optic neuritis who had failed to respond to conventional immunosuppressant therapy.61
Uveitic CME may fail to respond to immunosuppressive therapy.62 In such cases, topical difluprednate 0.05%, periocular triamcinolone acetonide, and intravitreal corticosteroid therapy such as the dexamethasone implant may be effective.63,64 Overall, the visual prognosis of patients with uveitis and MS is good compared to patients with idiopathic intermediate uveitis. Messenger et al23 found no statistically significant difference between patients with MS and intermediate uveitis and those with idiopathic intermediate uveitis.
Proper treatment of uveitic-associated complications, such as cataract and glaucoma surgery or pars plana vitrectomy with epiretinal membrane, is essential in maximizing long-term visual outcomes. Towler and Lightman30 reported a median visual improvement with cataract surgery or vitrectomy of 3 Snellen lines to a median visual acuity of 6/18. As with other forms of uveitic cataract, it is recommended that uveitis and macular edema be suppressed for at least several months prior to cataract surgery. Perioperative oral steroids65 or the intravitreal dexamethasone implant within 4 weeks before surgery66 are recommended. Occlusive retinal vasculitis with ischemia and retinal neovascularization should be treated with panretinal laser photocoagulation to help prevent vitreous hemorrhage, and pars plana vitrectomy is helpful if hemorrhage occurs.30
Patients with MS and uveitis should not be treated with fingolimod if at all possible because of a low but increased risk of macular edema (0.3% to 1.2%).34,35 If fingolimod is to be used for treatment of MS, an ophthalmic examination is recommended prior to initiating therapy and at regular intervals during treatment.
Interferon has been associated with a dose-dependent and reversible risk of retinal ischemia, causing cotton wool spots and retinal hemorrhages.67 Affected patients may not be symptomatic. However, severe proliferative retinopathy and vision loss with low-dose interferon has also been described.68
SUMMARY
Uveitis is the third most common ocular complication of MS after optic neuritis and oculomotor palsies. Large studies suggest that MS occurs in about 1% of patients, and uveitis occurs in about 1% of patients with MS. The uveitis may occur prior to, concurrent with, or after the diagnosis of MS. Intermediate uveitis is the most common manifestation, but anterior, posterior, and panuveitis all may occur. The diagnosis of MS in a patient with uveitis is best made in conjunction with a skilled neurologist. Topical, injected, and oral corticosteroids are the mainstay of treatment of uveitis and its sequelae, including macular edema, but immunosuppressive therapy may be necessary in patients intolerant or unresponsive to these drugs. In such cases, immunosuppressive therapy may be necessary; however, tumor necrosis factor inhibitors are contraindicated in the treatment of uveitis in patients with MS because they may potentiate demyelination. RP
REFERENCES
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387-A394.
- Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001;22(2):117-139.
- World Health Organization. Atlas multiple sclerosis resources in the world 2008 (PDF). Geneva: World Health Organization. pp. 15-16. Available at http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf .
- Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6(10):903-912.
- Compston A, Coles A . Multiple sclerosis. Lancet. 2008;372(9648): 1502-1517.
- National Multiple Sclerosis Society. Types of MS. Archived from the original on 7 July 2017. Retrieved 2 May 2018. Available at https://www.nationalmssociety.org/What-is-MS/Types-of-MS .
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636.
- McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
- Roemer S, Bissig A, Rocca A, Du Pasquier R, Guex-Crosier Y. Efficacy of natalizumab in intermediate uveitis related to multiple sclerosis: a case report. Klin Monbl Augenheilkd. 2018;235(4):476-477.
- Ciotti JR, Cross AH. Disease-modifying treatment in progressive multiple sclerosis. Curr Treat Options Neurol. 2018;20(5):12.
- Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
- Sanai SA, Saini V, Benedict RH, et al. Aging and multiple sclerosis. Mult Scler. 2016;22(6):717-725.
- Biousse V, Calvetti O, Drews-Botsch CD, et al; Optic Neuritis Survey Group. Management of optic neuritis and impact of clinical trials: an international survey. J Neurol Sci. 2009;276(1-2):69-74.
- Nerrant E, Tilikete C. Ocular motor manifestations of multiple sclerosis. J Neuroophthalmol. 2017;37(3):332-340.
- Olsen TG, Frederiksen J. The association between multiple sclerosis and uveitis. Surv Ophthalmol. 2017;62(1):89-95.
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-516.
- Miettinen R. Incidence of uveitis in Northern Finland. Acta Ophthalmol (Copenh). 1977;55(2):252-260.
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491-500.
- Jakob E, Reuland MS, Mackensen F, et al. Uveitis subtypes in a German interdisciplinary uveitis center - analysis of 1916 patients. J Rheumatol. 2009;36(1):127-136.
- Biousse V, Trichet C, Bloch-Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic-based series. Neurology. 1999;52(1):179-181.
- Le Scanff J, Sève P, Renoux C, Broussolle C, Confavreux C, Vukusic S. Uveitis associated with multiple sclerosis. Mult Scler. 2008;14(3):415-417.
- Messenger W, Hildebrandt L, Mackensen F, Suhler E, Becker M, Rosenbaum JT. Characterisation of uveitis in association with multiple sclerosis. Br J Ophthalmol. 2015;99(2):205-209.
- Shugaiv E, Tüzün E, Kürtüncü M, et al. Uveitis as a prognostic factor in multiple sclerosis. Mult Scler. 2015;21(1):105-107.
- Zein G, Berta A, Foster CS. Multiple sclerosis-associated uveitis. Ocul Immunol Inflamm. 2004;12(2):137-142.
- Steinlin MI, Blaser SI, MacGregor DL, Buncic JR. Eye problems in children with multiple sclerosis. Pediatr Neurol. 1995;12(3):207-212.
- Jordan JF, Walter P, Ayertey HD, Brunner R. Intermediate uveitis in childhood preceding the diagnosis of multiple sclerosis: a 13-year follow-up. Am J Ophthalmol. 2003;135(6):885-886.
- Sancho L, Kramer M, Koriat A, Eiger-Moscovich M, Sharon Y, Amer R. Complications in intermediate uveitis: prevalence, time of onset, and effects on vision in short-term and long-term follow-up. Ocul Immunol Inflamm. 2018 Jan 25:1-9.
- Engell T. Neurological disease activity in multiple sclerosis patients with periphlebitis retinae. Acta Neurol Scand. 1986;73(2):168-172.
- Towler HM, Lightman S. Symptomatic intraocular inflammation in multiple sclerosis. Clin Experiment Ophthalmol. 2000;28(2):97-102.
- Jouve L, Benrabah R, Heron E, Bodaghi B, Le Hoang P, Touitou V. Multiple sclerosis-related uveitis: does MS treatment affect uveitis course? Ocul Immunol Inflamm. 2017;25(3):302-307.
- Hedayatfar A, Falavarjani KG, Soheilian M, et al. Mycophenolate mofetil for the treatment of multiple sclerosis-associated uveitis. Ocul Immunol Inflamm. 2017;25(3):308-314.
- Cugati S, Chen CS, Lake S, Lee AW. Fingolimod and macular edema: pathophysiology, diagnosis, and management. Neurol Clin Pract. 2014;4(5):402-409.
- Zarbin MA, Jampol LM, Jager RD, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology. 2013;120(7):1432-1439.
- Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786-1793.
- Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68(18):1488-1494.
- Schmidt S, Wessels L, Augustin A, Klockgether T. Patients with multiple sclerosis and concomitant uveitis/periphlebitis retinae are not distinct from those without intraocular inflammation. J Neurol Sci. 2001;187(1-2):49-53.
- Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol. 2013;156(2):228-236.
- Petrushkin H, Kidd D, Pavesio C. Intermediate uveitis and multiple sclerosis: to scan or not to scan. Br J Ophthalmol. 2015;99(12):1591-1593.
- Smith JR, Rosenbaum JT. Neurological concomitants of uveitis. Br J Ophthalmol. 2004;88(12):1498-1499.
- Wakefield D, McCluskey P, Wildner G, et al. Inflammatory eye disease: Pre-treatment assessment of patients prior to commencing immunosuppressive and biologic therapy: Recommendations from an expert committee. Autoimmun Rev. 2017;16(3):213-222.
- Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17(5):467-480.
- Kuehn BM. Rare neurological condition linked to newer monoclonal antibody biologics. JAMA. 2009;301(14):1423-1424.
- Raja SC, Jabs DA, Dunn JP, et al. Pars planitis: clinical features and class II HLA associations. Ophthalmology. 1999;106(3):594-599.
- Zamecki KJ, Jabs DA. HLA typing in uveitis: use and misuse. Am J Ophthalmol. 2010;149(2):189-193.
- Optic Neuritis Study Group. The 5-year risk of MS after optic neuritis. Experience of the optic neuritis treatment trial. Neurology. 1997;49(5):1404-1413.
- Prieto JF, Dios E, Gutierrez JM, Mayo A, Calonge M, Herreras JM. Pars planitis: epidemiology, treatment, and association with multiple sclerosis. Ocul Immunol Inflamm. 2001;9(2):93-102.
- Gordon LK, Goldstein DA. Gender and uveitis in patients with multiple sclerosis. J Ophthalmol. 2014;2014:565262.
- Alshamrani F, Alnajashi H, Freedman M. Radiologically isolated syndrome: watchful waiting vs. active treatment. Expert Rev Neurother. 2017;17(5):441-447.
- Okuda DT. Radiologically isolated syndrome should be treated with disease-modifying therapy - Yes. Mult Scler. 2017;23(14):1818-1819.
- Okuda DT, Siva A, Kantarci O, et al; Radiologically Isolated Syndrome Consortium (RISC); Club Francophone de la Sclérose en Plaques (CFSEP). Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509.
- Labiano-Fontcuberta A, Benito-León J. Radiologically isolated syndrome should be treated with disease-modifying therapy - No. Mult Scler. 2017;23(14):1820-1821.
- Mehta S, Loevner LA, Mikityansky I, et al. The diagnostic and economic yield of neuroimaging in neuro-ophthalmology. J Neuroophthalmol. 2012;32(2):139-144.
- Shao H, Sun SL, Kaplan HJ, Sun D. Induction of autoimmune encephalomyelitis and uveitis in B6 and (B6×SJL) mice by peptides derived from myelin/oligodendrocyte glycoprotein. J Neuroimmunol. 2002;132(1-2):117-122.
- Li SY, Birnbaum AD, Goldstein DA. Optic neuritis associated with adalimumab in the treatment of uveitis. Ocul Immunol Inflamm. 2010;18(6):475-481.
- Cunningham ET Jr, Pavesio CE, Goldstein DA, Forooghian F, Zierhut M. Multiple sclerosis-associated uveitis. Ocul Immunol Inflamm. 2017;25(3):299-301.
- Donaldson MJ, Pulido JS, Herman DC, Diehl N, Hodge D. Pars planitis: a 20-year study of incidence, clinical features, and outcomes. Am J Ophthalmol. 2007;144(6):812-817.
- Becker MD, Heiligenhaus A, Hudde T, et al. Interferon as a treatment for uveitis associated with multiple sclerosis. Br J Ophthalmol. 2005;89(10):1254-1257.
- Mackensen F, Jakob E, Springer C, et al. Interferon versus methotrexate in intermediate uveitis with macular edema: results of a randomized controlled clinical trial. Am J Ophthalmol. 2013;156(3):478-486.
- Riera R, Porfírio GJ, Torloni MR. Alemtuzumab for multiple sclerosis. Cochrane Database Syst Rev. 2016;4:CD011203.
- Willis MD, Pickersgill TP, Robertson NP, Lee RW, Dick AD, Carreño E. Alemtuzumab-induced remission of multiple sclerosis-associated uveitis. Int Ophthalmol. 2016 Oct 11. [Epub ahead of print]
- Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002;10(4):239-246.
- Schallhorn JM, Niemeyer KM, Browne EN, Chhetri P, Acharya NR. Difluprednate for the treatment of uveitic cystoid macular edema. Am J Ophthalmol. 2018 Jul;191:14-22.
- Khurana RN, Porco TC. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina. 2015 Aug;35(8):1640-1646.
- Llop SM, Papaliodis GN. Cataract surgery complications in uveitis patients: a review article. Semin Ophthalmol. 2018;33(1):64-69.
- Larochelle MB, Smith J, Cacey MS. Dexamethasone intravitreal implant in the treatment of uveitic macular edema in the perioperative cataract setting: a case series. Am J Ophthalmol. 2016;166:149-153.
- Guyer DR, Tiedeman J, Yanuzzi LA, et al. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111(3):350-356.
- Savant V, Gillow T. Interferon-associated retinopathy. Eye (Lond). 2003;17(4):534-536.








