Fibrovascular membranes are the principal cause of tractional and combined tractional and rhegmatogenous retinal detachments (TRRDs) secondary to retinovascular disease.1,2 They are most commonly seen in eyes with proliferative diabetic retinopathy (PDR), but they can also be found as sequelae of vascular occlusions, sickle cell retinopathy, retinopathy of prematurity (ROP), and familiar exudative vitreoretinopathy (FEVR). The common predisposing factor is retinal ischemia causing vascular endothelial growth factor (VEGF)-mediated neovascularization.2 Fibrovascular membranes tend to occur at the interface of ischemic and nonischemic retina or at the optic nerve. In eyes with a posterior vitreous detachment, they can only grow on the surface of the retina; thus, retinal detachments do not tend to occur as sequelae. In younger eyes with attached vitreous gel, the posterior hyaloid acts as a scaffold that allows the fibrovascular tissue to grow, leading to traction on the retina and retinal detachments.
TYPES OF FIBROVASCULAR MEMBRANES
Fibrovascular membranes can range from extremely vascular to mostly fibrous, the latter being more common with increased duration, as the fibrous and contractile component increases over time.2 Fibrovascular membranes can start over the optic nerve in eyes with generalized ischemia, PDR, or central retinal vein occlusion (CRVO) or adjacent to vessels at the interface of ischemic and nonischemic retina. This onset is seen in PDR, branch retinal vein occlusions (BRVOs), sickle cell retinopathy, ROP, and FEVR. Membranes secondary to FEVR and sickle cell retinopathy tend to occur in the periphery, which makes their management more challenging. Fibrovascular membranes can also occur in the area of sclerotomies in ischemic eyes, as the fibrovascular ingrowth expands from the sclerotomies into the vitreous base, using the peripheral vitreous as a scaffold. This progression was more common in the era of larger sclerotomies and in eyes with neovascular glaucoma.
María H. Berrocal, MD, is director of Berrocal & Associates and professor of ophthalmology at the University of Puerto Rico School of Medicine and practices in San Juan, Puerto Rico. Luis A. Acaba, BA, is a medical student at the Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia, Pennsylvania. Dr. Berrocal reports personal fees from Alcon and Allergan. Mr. Acaba reports no related disclosures. Reach Dr. Berrocal at mariahberrocal@hotmail.com.
Editor’s note: This article is featured in a journal club episode of Straight From the Cutter’s Mouth: A Retina Podcast. Listen to the episode at www.retinapodcast.com .
PATHOPHYSIOLOGY
Fibrovascular membrane formation is the hallmark of PDR. Inflammation and angiogenesis induced by ischemia cause fibrovascular membrane growth at the retinovitreal interface. Fibrovascular membranes are formed by migration and replication of fibroblasts, glial cells, macrophages, monocytes, pericytes, and vascular endothelial cells.1 Myofibroblasts differentiated from fibroblasts derived from monocytes result in contraction of the fibrovascular membrane and traction retinal detachment (TRD).2
TRACTIONAL RETINAL DETACHMENT
Tractional retinal detachment is a common indication for pars plana vitrectomy. It occurs more commonly in young diabetic patients with proliferative disease. Traditional indications for surgery were retinal detachment involving or threatening the fovea. Currently, the indications for surgery can be expanded to severe disease or significant traction since observation alone can result in increased traction tearing the retina, resulting in a TRRD.3,4 Prior to microincisional vitrectomy, membrane dissection techniques involved the use of bimanual techniques, scissors, forceps, picks, and viscodissection since the vitreous cutter could only remove tissue distant from the retina. Fibrovascular tissue had to be separated from the retina, either by segmentation or by delamination with instruments since bringing the vitreous cutter close to the retina resulted in iatrogenic breaks. The advent of small-gauge vitrectomy platforms with up to 10,000 cpm and optimized fluidic control have made possible the development of novel techniques for fibrovascular membrane removal. This evolution has simplified the surgical management of complex cases, reduced the need for ancillary instrumentation and bimanual surgery, and reduced complications.
SURGICAL TECHNIQUE
In our institution, preoperative anti-VEGF injection is performed in cases in which the membranes are very vascular or the patient has poorly controlled hypertension or is on anticoagulation.5,6 The injection should be performed ideally between 2-5 days preoperatively and once a medical clearance is obtained.6 Severe contraction of fibrovascular tissue with worsening detachments has been described in eyes with TRDs when anti-VEGF drugs were injected 7 days or longer prior to surgery.7,8
Valved cannulas are most useful as they provide improved intraocular pressure (IOP) control during the surgical procedure, thus facilitating hemostasis during surgery. Awareness and optimization of the patient’s systemic blood pressure are important because they also minimize intraoperative bleeding. The vitrectomy machine settings are maximum linear aspiration, controlled with the foot pedal by the surgeon, and the maximum cutting speed available for the probes (5,000-10,000 cpm), ideally 10,000 cpm. In our experience, IOP control is set at 30 mmHg but can be lowered in eyes with glaucomatous damage or raised during the surgery to control bleeding. The most difficult pathologies are best managed by the smallest gauges, namely 27-gauge, since the smaller vitrectomy probes can move between the retina and the fibrovascular tissue and allow for more control of membrane removal.9
MEMBRANE DISSECTION: LIFT AND SHAVE AND INSIDE-OUT DISSECTION
The lift and shave technique is an all-probe membrane removal technique that makes optimal use of 25- and 27-gauge instruments.10 It can efficiently remove fibrovascular tissue in both TRDs and TRRDs with minimal use of ancillary instruments. Most surgical cases can be performed with probe-only membrane dissection. A core vitrectomy is performed with removal of the peripheral vitreous skirt using the vitrectomy probe. An open tissue plane is found between the membranes and the retina, and the vitrectomy probe is advanced at the same time as blunt dissection is performed laterally. Back-cutting is performed at maximum cutting speeds as the probe is withdrawn with the port facing anteriorly. This action segments the dissected tissue. The edges of membranes are lifted with the cutter and suction, and once resistance is met, the lifted tissue is cut. A combination of blunt dissection, back-cutting, and sequential, alternating suction and cutting is used to segment all of the fibrovascular tissue. Remaining stumps can be shaved safely from the surface of the retina or vessels with the cutter at maximum cutting speeds. On occasion, there is no potential plane available to introduce the vitrectomy probe because the fibrovascular tissue is tightly adherent throughout the peripheral edges of the membrane along the arcades. In these cases, we perform an inside-out dissection, which consists of detaching the fibrovascular tissue from the optic nerve, either with high suction and the probe or with forceps. Once this tissue is separated from the optic nerve, it is removed with the cutter, and dissection planes are made available around the optic nerve. The same lift and peel techniques are utilized to remove fibrovascular membranes, but from the center to the periphery (Figure 1).
Bleeding during membrane dissection can be controlled by increasing the IOP to 60 mmHg; if doing so does not stop bleeding, diathermy can be applied to the bleeding vessel with caution not to occlude it completely. Alternately, continuous laser can be used as diathermy, or direct pressure on the bleeder can be applied using the vitrectomy probe. This latter maneuver is particularly useful in bleeders on vessels in the optic nerve head. It is important to only leave the IOP raised for as short a time as possible to secure hemostasis, as a prolonged increase in IOP can cause optic nerve damage.
In cases of TRDs in which iatrogenic breaks are not created, it is not necessary to remove all of the fibrovascular membranes, as nasal membranes can be left alone and remain stable.11 However, it is imperative to remove the posterior hyaloid, particularly around any fibrovascular tissue that will be left, because this tissue can serve as a scaffold and progression of the detachment can ensue in the postoperative period. After all of the membrane dissection is done, remaining blood is aspirated from the retinal surface, and laser photocoagulation is applied to the peripheral retina and attached retina in panretinal photocoagulation (PRP) fashion. Fluid can be left in the eye, or in cases in which there was traction in the posterior pole, an air tamponade can be left in the eye and the patient positioned prone for 1 day to help resolve the subretinal fluid more rapidly.
TRRDs are managed with similar techniques, except that the bullously detached retina renders membrane removal more challenging, and total fibrovascular tissue removal is necessary for reattachment.12-15 Finding a plane to begin dissection can be more difficult, and the inside-out technique is most useful in these cases. Although most cases can be tackled unimanually and the dissection of membranes performed with the probe, very adherent membrane removal can sometimes require viscodissection with hyaluronic acid or bimanual techniques with chandelier illumination. Viscodissection can be performed through a retractable cannula that is available in small gauges to fit through 25- and 27-gauge cannulas. Once a plane is found, the viscoelastic is injected to separate the membranes from the retina. As the injection is performed, blunt dissection with the injection cannula can also be done. Removal of tissue with the cutter is then performed. Our preferred bimanual technique involves placing a chandelier at 12 o’clock, using forceps in the nondominant hand to grasp the edge of membranes, and using the cutter in the dominant hand to perform blunt dissection or segmentation. Rarely are scissors necessary because the small 25- and 27-gauge cutters can act as scissors. The highest cutting rates are used since doing so minimizes movement of the detached retina and prevents iatrogenic breaks.
Once all of the fibrovascular tissue is removed, breaks are marked with diathermy to aid in their visualization under air. An air-fluid exchange is performed with drainage through the breaks and aspiration, either with the cutter in aspiration mode or with a soft-tip cannula. Once all subretinal fluid is removed, laser is applied around breaks in a PRP fashion. We prefer a gas tamponade, utilizing C3F8 for eyes with many breaks and C2F6 for eyes with mostly superior breaks. Patients are instructed to maintain prone positioning for 1-2 weeks.
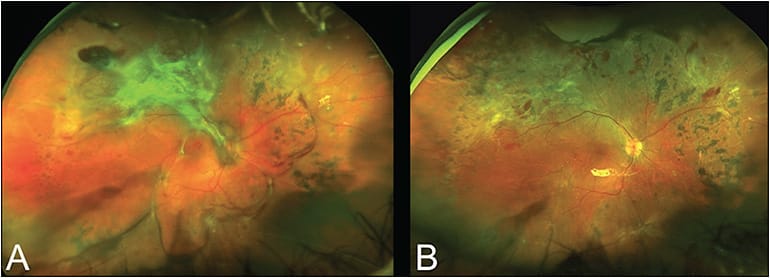
Silicone oil is utilized in patients who cannot position, monocular patients, or patients who are required to travel by air. Disadvantages of the oil are that, if bleeding occurs in the perioperative period, blood clots become trapped on the surface of the retina, and fibrous membranes can form, as well as the need for a second operation for oil removal. Excellent anatomic results can be obtained with 27-gauge vitrectomy in TRRDs, utilizing the described dissection techniques (Figure 2).15

POSTOPERATIVE CARE
The most common postoperative complications of surgery include hypotony, bleeding, and redetachment. Hypotony on the first day is avoided by optimal wound construction and checking the sclerotomies for optimal closure at the end and suturing them when necessary. Bleeding in the postoperative period can be managed with observation if mild or with an air-fluid exchange in the office. Reduced rates of hypotony and rebleeding are seen with 27-gauge, most likely because of the optimal wound closure with this smallest gauge.9,16 Redetachment occurs most commonly in TRRDs and is the result of poor positioning, inadequate gas fill, missed breaks, residual fibrovascular tissue, or reproliferation.17
FUTURE CONSIDERATIONS
Retinal detachments secondary to retinal vascular disease are a leading cause of blindness, particularly among working-age patients with diabetes. Prevention of ischemia with optimal diabetic control is ideal but not always achievable. Detachment of the vitreous eliminates the scaffold that neovascularization can utilize to grow into the vitreous and can cause traction on the retina. Safely detaching the vitreous in patients with vascular disease, particularly those with diabetes, can prevent tractional detachments in this population. Pharmacologic interventions in this area are being studied and could hold promise. Performing early vitrectomy, prior to the development of a tractional component, can also prevent the development of TRDs. A small series showed a 4-fold reduction in severe visual loss with early vitrectomy in eyes with PDR in diabetic patients younger than 50 years of age.18 Given the reduced complications obtained in vitrectomy surgery with technological advances over the last decade, early vitrectomy as a treatment modality can be considered in young diabetic patients with proliferative disease.15,19 RP
REFERENCES
- Snead DR, James S, Snead MP. Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye. 2008;22:1310-1317.
- Tamaki K, Usui-Ouchi A, Murakami A, Ebihara N. Fibrocytes and fibrovascular membrane formation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57:4999-5005.
- Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial: Diabetic Retinopathy Vitrectomy Report 4. The Diabetic Retinopathy Vitrectomy Study Research Group. Ophthalmology. 1988;95:1321-1334.
- Flynn HW Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL 3rd. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99:1351-1357.
- Yeoh J, Williams C, Allen P, et al. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Exp Ophthalmol. 2008;36:449-454.
- Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927-938.
- Arevalo JF, Sanchez JG, Saldarriaga L, et al. Retinal detachment after bevacizumab. Ophthalmology. 2011;118(11):2304.e3-e7.
- Arevalo JF, Maia M, Flynn HW Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213-216.
- Khan MA, Shahlaee A, Toussaint B, et al. Outcomes of 27 gauge microincision vitrectomy surgery for posterior segment disease. Am J Ophthalmol. 2016;161:36-43.
- Berrocal MH. All-probe vitrectomy dissection techniques for diabetic tractional retinal detachments: lift and shave. Retina. 2017 Oct 11. [Epub ahead of print]
- Charles S, Flinn CE. The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol. 1981;99:66-68.
- Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous retinal detachment. Arch Ophthalmol. 1987;105:503-507.
- Yang CM, Su PY, Yeh PT, Chen MS. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: clinical manifestations and surgical outcome. Can J Ophthalmol. 2008;43:192-198.
- Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic rhegmatogenous retinal detachment. Am J Ophthalmol. 1983;95:34-44.
- Cruz-Iñigo YJ, Berrocal MH. Twenty-seven-gauge vitrectomy for combined tractional and rhegmatogenous retinal detachment involving the macula associated with proliferative diabetic retinopathy. Int J Retina Vitreous. 2017;3:38.
- Khan MA, Kuley A, Riemann CD, et al. Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior-segment disease. Ophthalmology. 2018;125:423-443.
- Schrey S, Krepler K, Wedrich A. Incidence of rhegmatogenous retinal detachment after vitrectomy in eyes of diabetic patients. Retina. 2006;26:149-152.
- Berrocal MH, Acaba LA, Acaba A. Surgery for diabetic eye complications. Curr Diab Rep. 2016;16:99.
- Cruz-Iñigo YJ, Acabá LA, Berrocal MH. Surgical management of retinal diseases: proliferative diabetic retinopathy and traction retinal detachment. In: Oh H, Oshima Y, eds. Microincision Vitrectomy Surgery, vol. 54. Basel, Switzerland: Karger Publishers; 2014:196-203.








