FDA Approves First AI Device to Detect Diabetic Retinopathy
IDX-DR scored well in clinical trial.
■ The FDA has approved marketing of the IDx-DR (IDx), the first medical device to use artificial intelligence to detect greater than a mild level of diabetic retinopathy in adults.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy since about 50% of them do not see their eye doctor on a yearly basis,” said Malvina Eydelman, MD, director of the Division of Ophthalmic and Ear, Nose, and Throat Devices at the FDA’s Center for Devices and Radiological Health. “(This) decision permits the marketing of a novel artificial intelligence technology that can be used in a primary care doctor’s office.”
The device is based on a software program that uses an artificial intelligence algorithm to analyze images of the eye taken with a Topcon NW400 retinal camera. A doctor uploads the digital images of the patient’s retinas to a cloud server on which IDx-DR software is installed. If the images are of sufficient quality, the software provides the doctor with one of two results: (1) “more than mild diabetic retinopathy detected: refer to an eye care professional” or (2) “negative for more than mild diabetic retinopathy; rescreen in 12 months.” If a positive result is detected, patients should see an eye care provider for further diagnostic evaluation and possible treatment as soon as possible.
IDx-DR is the first device authorized for marketing that provides a screening decision without the need for a clinician to also interpret the image or results, which makes it usable by health care providers who may not normally be involved in eye care.
The FDA evaluated data from a clinical study of retinal images obtained from 900 patients with diabetes at 10 primary care sites. The study was designed to evaluate how often IDx-DR could accurately detect patients with more than mild diabetic retinopathy. In the study, IDx-DR was able to correctly identify the presence of more than mild diabetic retinopathy 87.4% of the time and was able to correctly identify those patients who did not have more than mild diabetic retinopathy 89.5% of the time.
Study Conducted of Adverse Events Following Vitrectomy
Macular surgery does not require intensive follow-up.
■ Researchers from the Department of Ophthalmology at the Byers Eye Institute, Stanford University Medical Center, Palo Alto, California, sought to evaluate the utility of standard postoperative visit (POV) intervals in pars plana vitrectomy (PPV) as a function of adverse events (AEs) identified.
A retrospective chart review was conducted on all patients undergoing PPV for any indication from January 1, 2016, to December 31, 2016. Baseline data and postoperative AEs were collected from the preoperative and POVs. Each POV was assessed as either a scheduled (s-POV), physician adjusted (a-POV), or patient-initiated visit (p-POV) which were used to categorize the patients. Additionally, the preoperative features, diagnoses, and surgical procedures were evaluated to determine protective factors and risk factors for AEs.
A total of 256 patients (310 PPVs) met inclusion criteria. The most common cumulative postoperative AEs were elevated intraocular pressure (>30 mmHg) (12.3%), cystoid macular edema (6.1%), and retinal detachment (5.8%). Patients with the diagnosis of macular hole or epiretinal membrane had the lowest relative risk of AEs, while patients with retinal detachment had the highest relative risk. There was no difference in time-to-AE between 23-, 25-, and 27-gauge vitrector. Patients in a-POV and p-POV had a statistically higher incidence of AEs in the postoperative day 5 to postoperative day 10 window.
The researchers, who presented their findings at the recent ARVO meeting, concluded that the incidence of adverse events following isolated macular surgery (ERM peel or macular hole repair) was very low and therefore may warrant a less intensive follow-up regimen.
Brolucizumab Is Reliable for 12-Week Dosing
Once started, most patients maintained that regimen.
■ Wet AMD patients who initially demonstrate that they can be treated with the Novartis investigational anti-VEGF drug brolucizumab every 12 weeks are likely to be able to maintain that quarterly treatment interval at least through the first year. These new and positive brolucizumab (RTH258) data come from a secondary analysis of the phase 3 HAWK and HARRIER trials.
This is the first time a high level of reliability has been prospectively demonstrated for a prespecified secondary endpoint of a 12-week dosing interval with an anti-VEGF therapy in phase 3 trials. These additional data were presented at the ARVO 2018 Annual Meeting. Last June, the company announced positive results from these 2 phase 3 studies of brolucizumab, showing noninferiority to aflibercept in mean change in BCVA from baseline to week 48.
Previously reported findings demonstrated that a majority of brolucizumab 6 mg patients in the trials - 57% in HAWK and 52% in HARRIER - were maintained on a 12-week dosing interval following the loading phase through week 48. The new findings showed that brolucizumab 6 mg patients who were suitable for 12-week treatment intervals during the first 12-week cycle after the loading phase had an 87% (HAWK) and 83% (HARRIER) probability of remaining on this quarterly treatment interval through week 48. The ability to reliably assess the likelihood of patients remaining on quarterly dosing could help physicians and patients better manage, personalize, and optimize treatment plans.
“The ability to quickly identify patients who can maintain a 12-week interval has the potential to simplify treatment plans for wet AMD patients,” said Glenn J. Jaffe, MD, chief of retinal ophthalmology at Duke University in Durham, North Carolina, and an author of the presentation in a news release. “These robust data may offer physicians confidence that when 12-week dosing with brolucizumab is initially successful, there is high probability that the patient will maintain this interval through the first year of treatment.”
In addition to brolucizumab, Eylea (Regeneron), abicipar pegol (Allergan), Luminate (Allegro), and RG7716 (Genentech) have all shown in various clinical trials that they could be effective in extended, 12-week dosing regimens.
Comparison Between 25-Gauge and 27-Gauge RD Repair
Slight differences found in 129-patient study.
■ Researchers at the University of Electronic Science and Technology, Chengdu, Sichuan, China, sought to retrospectively compare the safety and effectiveness of 27-gauge microincision vitrectomy surgery (MIVS) with 25-gauge MIVS for treating primary rhegmatogenous retinal detachment (RRD).
One hundred and twenty-nine eyes of 129 patients with RRD underwent MIVS from May 1, 2015, to June 31, 2017 and were included in this study. Eighty-four eyes underwent 25-gauge vitrectomy and 45 eyes for 27-gauge vitrectomy, respectively. The analysis included surgical time, main clinical outcomes, and rate of complications.
The mean surgical times was 69.9±41.2 minutes for the 25-gauge group and 61.7±34.1 minutes for the 27-gauge group, and there were no significant difference between the 2 groups. The primary anatomical success rate after a single operation was 96.4% and 91.1% for the 25-gauge group and 27-gauge group, respectively.
Postoperative BCVA increased significantly in both groups. However, there were no significant differences in terms of visual improvement between the 2 groups. No severe intraoperative complication was observed. Iatrogenic retinal breaks occurred in 2 eyes (2.4%) in the 25-gauge group and 1 eye (2.2%) in the 27-gauge group during the peripheral vitreous base shaving.
The researchers, who presented their findings at the recent ARVO meeting, concluded that the study found no significant anatomical or functional difference between 27-gauge PPV and 25-gauge PPV in the treatment of primary RRD. Therefore, 27-gauge vitrectomy would be a safe and effective surgery for repairing primary RRD.
AGTC Advances Two Key Trials
Gene therapy for inherited retinal diseases.
■ Applied Genetic Technologies Corporation (AGTC), which is conducting clinical trials using its proprietary adeno-associated-virus (AAV)-based gene therapies to treat rare, inherited retinal diseases, said it has dosed the first patient in what is planned as a 15-patient phase 1/2 trial for X-linked retinitis pigmentosa (XLRP). The replacement gene was administered intravitreally in a single-injection procedure. The company also announced that it has completed enrollment in what is planned as a 27-patient phase 1/2 study for X-linked retinoschisis (XLRS).
XLRP is an inherited condition that causes progressive vision loss in boys and young men. Characteristics of the disease include night blindness in early childhood and progressive constriction of the visual field. In general, XLRP patients experience a gradual decline in visual acuity over the disease course, which results in legal blindness around the fourth decade of life. Preclinical data in a canine model of XLRP caused by mutations in the RPGR gene indicate that treatment with an AAV-based gene therapy product slowed the loss of visual function.
XLRS is an inherited early-onset retinal degenerative disease and the leading cause of juvenile macular degeneration in males. Characterized by abnormal splitting of the layers of the retina, the disease begins early in childhood and causes poor visual acuity in young boys, which may materialize into legal blindness in adulthood. Affected boys typically have best-corrected visual acuity of 20/60 to 20/120 at initial diagnosis. Severe complications such as retinal hemorrhage or retinal detachment occur in up to 40% of patients, especially in older individuals.
Both the XLRP and XLRS programs are part of AGTC’s collaboration with Biogen. Under the terms of the collaboration, AGTC will receive a milestone payment of $2.5 million as a result of enrollment of the first patient in the XLRP trial.
NEI to Study 500 Early-Stage AMD Patients
Goal is to identify biomarkers of disease progression.
■ More details have emerged regarding a new clinical study led by the National Eye Institute (NEI), part of the National Institutes of Health. The study will follow 500 people over 5 years to learn more about the natural history of early-stage AMD. By using the latest technologies to visualize structures within the eye and measure their function, researchers hope to identify biomarkers of disease progression.
“The findings will contribute to our understanding of the underlying biology driving the transition from early to late-stage disease so that therapies can be developed to halt its progression,” said the study’s lead investigator, Emily Y. Chew, MD, deputy clinical director at NEI and director of the NEI Division of Epidemiology and Clinical Applications. “We want to better leverage advances in genetics, imaging and visual functioning tests so we can look at early-stage disease with more granularity. There may be surrogate markers of an individual’s risk of developing late-stage disease long before the disease progresses,” she said.
The AMD Ryan Initiative Study (ARIS) will track the eye health of 200 people who have bilateral early AMD, defined by the presence of medium-size drusen, yellowish deposits that accumulate under the retina. In addition, ARIS will include 200 people with early, reticular pseudodrusen, a type of lesion that causes a giraffe-like macular pattern. The composition and location of the reticular pseudodrusen differ from that of typical drusen. Some data suggest reticular pseudodrusen are associated with a higher than usual risk for progression to late disease, but more research is needed about this group. The study will enroll 100 age-matched, drusen-free control participants.
All participants will undergo routine spectral domain optical coherence tomography (SD-OCT), which is sensitive enough to detect even small changes in the volume of drusen over time. In addition, visual function will be measured by dark-adapted fundus perimetry, a test that measures the sensitivity of light perception in specific parts of the retina after a person’s eyes have adapted to the dark. Another visual function test, dark adaption, is useful for evaluating night vision impairment.
IN BRIEF
Research and industry news in retina.
BY JERRY HELZNER, CONTRIBUTING EDITOR
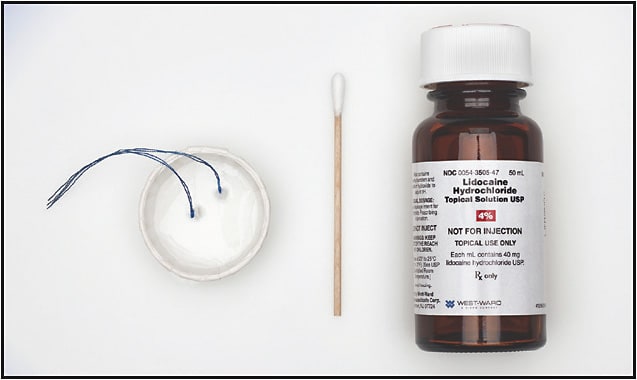
Numbing Product Launched for Intravitreal Injections
■ American Surgical Company (ASC), a specialty medical device manufacturer, has launched its Ophthalmic Anesthesia Comfort Pack, allowing for a more comfortable and less traumatic application of a numbing agent to the eye prior to an intravitreal injection. A 4% lidocaine solution is applied to the eye using uniform manufactured strung cotton pledgets designed and packaged for sterile placement in and retrieval from the fornix.
Michael McAllister, MD, who developed the product with ASC, says the Comfort Pack reduces patient stress. “Since I began using the Comfort Pack, my patients consistently tell me that my injections are some of the most comfortable they have experienced.”
Conventional numbing techniques utilize either an initial subconjunctival injection of a lidocaine solution or the application of a topical gel. According to the company, lidocaine injection can present risks such as poor visibility of the injection site secondary to subconjunctival hemorrhage, and the viscosity of a topical gel can limit the penetration of the antiseptic solution.
Ocular Cancer “Outbreaks” Require Further Study
■ Recent reports in the mainstream press focus on 36 Auburn University alumni and 18 young adults in North Carolina who have either been diagnosed with ocular cancer or self-reported that they have been afflicted. The normal incidence of ocular cancer is 6 in 1 million each year. Retinal Physician asked Carol Shields, MD, chief of ocular oncology at Wills Eye Hospital and a world-renowned authority on the disease for comment.
“We first heard of a potential ‘cancer cluster’ several years ago in North Carolina, but the state and CDC studied the situation and concluded that this was not likely a cluster. Several of the patients were treated by our team in Philadelphia and there was specific inquiry into environmental or genetic relationship, which proved negative. Much of this cluster was attributed to rapid communication through social media. Then the Auburn cluster was noted, but still there is no epidemiologic evidence of a true cluster and not all of the cases are validated. Again, some are a result of social media.”
Dr. Shields said the most curious part of the story is that many of the patients are fairly young women and men, noting that further investigation into this unusual situation is warranted.
ThromboGenics Begins Combination DME Study
■ ThromboGenics NV has enrolled the first patient in a phase 2 active-controlled, masked, multicenter study to evaluate the efficacy and safety of THR-317 administered in combination with ranibizumab (Lucentis; Genentech). THR-317 is a recombinant humanized monoclonal antibody directed against the receptor-binding site of human placental growth factor (PlGF) administered by intravitreal injection. Anti-PlGF has been shown in preclinical models to have antiangiogenic properties and to be anti-inflammatory.
This phase 2 study is designed to evaluate the safety and efficacy of THR-317 along with ranibizumab, compared with ranibizumab monotherapy in patients with DME. Patients will be randomized into either a combination arm of THR-317 (8mg) plus ranibizumab, or ranibizumab plus a sham administration. The primary outcome measure is change from baseline in BCVA at month 3.
ThromboGenics theorizes that simultaneous inhibition of VEGF (by ranibizumab) and PlGF (by THR-317) may have a better efficacy than either treatment alone. Nonclinical experiments indicate that anti-PlGF in the presence of an anti-VEGF antibody has an additive effect, inhibiting the growth of new blood vessels. This may mean that a combination approach could result in a better treatment response. The anti-PlGF component could bring the advantage of reduced inflammation associated with a reduced level of PIGF.
Approximately 70 patients will be enrolled, of which about half will be anti-VEGF treatment naïve and the other half will have had a suboptimal response to prior treatment with ranibizumab. Initial results from the THR-317-002 study are anticipated by Q3 2019.
Photobiomodulation Therapy Effective in Reducing Drusen
■ LumiThera Inc., a clinical-stage medical device company focused on developing noninvasive photobiomodulation (PBM) therapies for ocular disorders and disease, presented positive topline final results from the LIGHTSITE I clinical trial for the treatment of dry AMD utilizing its LT-300 Light Delivery System.
The LIGHTSITE I data were presented at the recent ARVO meeting by Marion Munk, MD, PhD, Department of Ophthalmology/Bern Photographic Reading Center, University Hospital Bern in Bern, Switzerland. Dr. Munk was part of the investigator team.
“LumiThera PBM treatment demonstrated reductions in central drusen volume over the course of the 1-year study vs the sham treatment with statistical significance at 1 year. Drusen is the hallmark pathology of dry AMD and is an important proinflammatory mediator and marker for disease progression,” said Dr. Munk in a news release.
“Results from the LIGHTSITE I study following treatment with a multiwavelength PBM treatment demonstrated clinical improvements in vision outcome measures providing a strong foundation for initial therapy as well as the need for follow-up maintenance therapy,” stated Samuel Markowitz, MD, co-principal investigator, Department of Ophthalmology and Vision Sciences, University of Toronto, in a news release. “The PBM therapy was most beneficial in dry AMD patients immediately following the completion of the treatment sessions. Contrast sensitivity or detailed vision was significantly improved throughout the year. Retreatments at a 6-month interval were performed to maintain clinical benefits.”
Kodiak Sciences Raises $33 Million for Novel Anti-VEGF
■ Kodiak Sciences Inc., a development-stage biopharmaceutical company specializing in novel therapeutics to treat high-prevalence ophthalmic diseases, has announced the completion of a $33 million mezzanine private financing of convertible notes. Kodiak intends to use the proceeds to advance the development of its lead product candidate, KSI-301, an anti-VEGF therapy for wet AMD and diabetic retinopathy.
Kodiak says its lead product candidate, KSI-301, is a novel pre-IND stage anti-VEGF biologic therapy designed to combine inhibition of a known pathway with a potentially superior ocular durability profile compared to drugs currently marketed for wet AMD and DR. KSI-301 is built with Kodiak’s antibody biopolymer conjugate platform, which is designed to maintain potent and effective drug levels in ocular tissues. By addressing the primary causes of undertreatment, Kodiak says KSI-301 has the potential to improve and sustain visual acuity outcomes in patients with neovascular conditions of the retina, such as wet AMD and DR.
MeiraGTx Gets Fast-Track Designation for Gene Therapy
■ MeiraGTx Limited, a clinical-stage gene therapy company, said the FDA has granted fast-track designation to AAV-RPGR for treatment of X-linked retinitis pigmentosa (XLRP) due to defects in the retinitis pigmentosa GTPase regulator (RPGR) gene.
MeiraGTx is currently conducting a dose-escalation clinical trial of AAV-RPGR in adult and pediatric patients diagnosed with XLRP caused by mutations in the eye-specific form of the RPGR gene. AAV-RPGR has also received orphan drug designation from the FDA. In addition to the phase 1/2 study, MeiraGTx is continuing to conduct an ongoing natural history study of patients with XLRP.
INSIDE RETINA BUSINESS
Recruiting: Be Thorough for Best Fit
By Jerry Helzner, contributing editor
With the demand for retina services continuing to grow, more practices will be thinking of adding a new physician to their staff. Practice owners should anticipate the need to hire a new physician a year in advance of the hire. This allows adequate time for recruitment and credentialing and avoids stress on the practice from increased patient demand.
SITE VISITS
Doing a site visit can lead to the best possible choice of a new employee. The site visit provides an opportunity to watch a candidate treating patients and to observe how the physician conducts him- or herself.
“It’s expensive for a partner to go on the road for a day or two to assess a finalist candidate,” says Amir Arbisser, MD, a founder of Eye Surgeons Associates, with offices in Iowa and Illinois, “but it’s much cheaper than signing the wrong doctor, credentialing them and moving their family to town, introducing them to patients and referral sources, and then having to legally extricate them months or years later, he says. “You are going to spend more time with this person than with your spouse. We are willing to make the investment to make sure that we make the right choice.”
HIRING PRIORITIES
Of all the priorities that go into a hiring decision, most important is that the candidate is a good fit with the practice culture. Reviewing a candidate’s social media and web presence can provide insight toward this goal.
Location may also be an important factor for the candidate, especially if the potential new hire has a young family and a spouse with a career, which is generally the case. Opportunities for the spouse to obtain meaningful employment near your location will win points from your potential hire.
Dr. Arbisser advises practices in hiring mode “to recruit the spouse to the community and to arrange appropriate opportunities and interviews.” Obtaining the services of a realtor to talk with candidates about nearby schools and neighborhoods can also help candidates make an informed decision.
COMPENSATION
You will usually not lose a good candidate over compensation, but most practice owners agree that a new physician should be given guaranteed compensation for at least the first year.
“We provide a guarantee and fairly low patient volume for the first year,” says Dr. Arbisser. “We want the new doctor to spend time enhancing the patient experience with no distractions. With patient care as the sole focus, the doctor begins to earn a positive reputation in the community.”
Once the new doctor is on board, give him or her every opportunity to become known in the community. Announce the hire in the newspaper. Hold “meet and greets.” Introduce the doctor to the local media, offering opportunities for him or her to do interviews and provide helpful articles on eye care.








