Optical coherence tomography angiography (OCTA) is a novel imaging technology that is currently being promoted for clinical use. This far, there are at least 5 companies with OCTA devices (Zeiss, Optovue, Topcon, Heidelberg Engineering, and Nidek) with Zeiss and Optovue currently FDA approved in the United States. OCTA is an extension of OCT technology, with which all retinal specialists are familiar.
The clinical use of structural OCT B scans has revolutionized clinical ophthalmology, especially for diagnosis of macular disease and evaluation of retinal therapeutics. OCTA adds a new dimension to diagnostic imaging with its ability to image flow in the retinal and choroidal vasculature. OCTA images can be segmented to give an en face, depth-encoded slab of the vasculature that is coregistered with the accompanying structural OCT B scan. OCTA imaging provides exquisitely detailed flow images and can image the deep retinal vascular plexus and choriocapillaris, which are not defined with fluorescein angiography (FA).
In my practice, OCTA has replaced FA for diagnosis of most retinovascular disorders and choroidal neovascularization (CNV). This article provides some practical tips that may ease the transition when incorporating OCTA into clinical practice as well as facilitate image interpretation.
LEARNING CURVE
Whenever a new technology finds its way into the clinic, expect a learning curve and be patient. This applies to the technician learning to use the device as well as the physician with respect to interpretation. It takes only 2 to 3 minutes to acquire a series of bilateral OCTA images. It is safe without the potential for allergic fluorescein dye reactions. However, there are 2 potential limitations to OCTA image interpretation that the clinician should be familiar with: artifacts and segmentation. Familiarity with these limitations will help to overcome pitfalls in image interpretation.
Because OCTA depicts flow that is inferred by movement of red blood cells between successive OCT scans, OCTA imaging is very sensitive to “motion artifacts” induced by patient motion (such as head movement, loss of fixation, and even microsaccades). Projection artifact refers to erroneous imaging of superficial retinal vessels onto the deeper retinal image.1 OCTA devices automatically segment images into slabs of the superficial retinal capillary plexus, deep retinal capillary plexus, outer retina, and choriocapillaris. The borders of these layers may differ slightly depending on the OCTA device used.
For the Optovue Avanti software version at the time of this article, the superficial capillary plexus upper border is at 3 µm below the internal limiting membrane (ILM) to the lower border at 15 µm below the inner plexiform layer (IPL). The deep capillary plexus spans from 15 µm below the IPL to 70 µm below the IPL. The outer retinal layer spans from this to 30 µm below the retinal pigment epithelium (RPE). The choriocapillaris spans from 30 µm to 60 µm below the RPE.
Segmentation errors may occur in the presence of retinal pathology, and the operator may need to manually adjust the automated segmentation lines and sequentially observe images to evaluate for retina pathology. OCTA devices have software to adjust for potential artifacts. In order to compensate for this, consider initially focusing OCTA imaging on patients with retinovascular disease or suspected CNV who are able to fixate.
IMAGE INTERPRETATION
OCTA provides a static detailed representation of flow without dye injection in contract to the dynamic, less detailed image after dye injection in FA. The OCTA image is accompanied by a co-registered structural OCT B scan evaluating cross sectional retinal anatomy. OCTA imaging depicts an en face, segmented slab of retinal flow, introducing this newer terminology into clinical use. Segmentation places boundary lines on the OCT B scan to delimit a slab of flow on the OCTA image. Flow signal overlay on the OCT B scan further confirms the presence on flow on the OCTA image.
To accurately interpret pathology, be familiar with normal anatomy on OCTA imaging (Figure 1). For retinovascular disorders, focus on the superficial and deep capillary plexus scans. The en face infrared image can be useful to show subtle intraretinal cysts which appear as black circular or oval voids, typically in the deep retinal plexus slab (Figure 2). This is in contrast to retinal nonperfusion, which appears as lack of vascular perfusion or vascular cut-off in a region where flow should be present.2

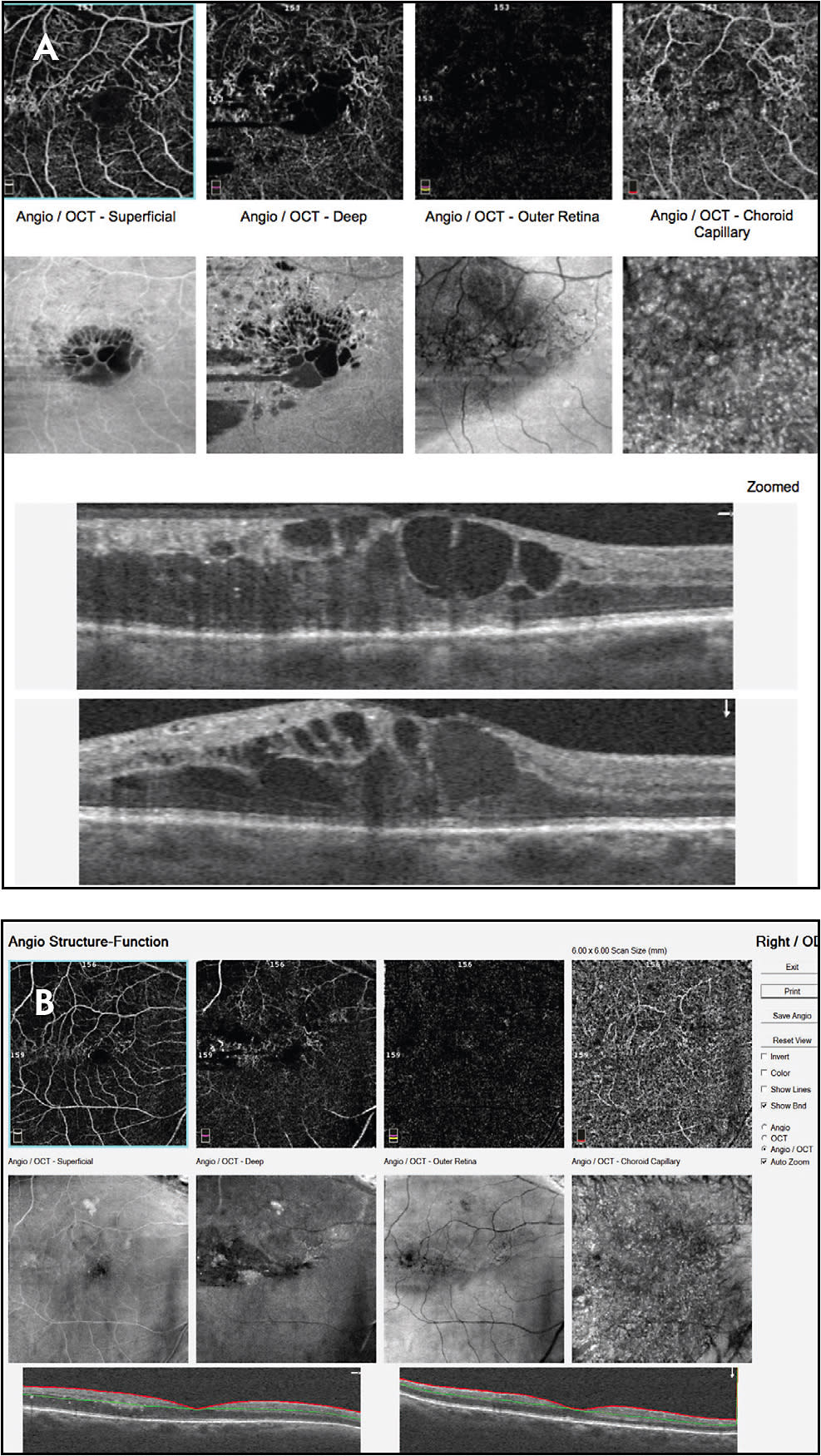
For CNV, evaluate the outer retinal and choriocapillaris scans (Figure 3). Manual adjustment of the automated segmentation lines may be necessary if CNV is not evident on initial view of machine generated readout (Figure 4). The 3 mm x 3 mm scans provide the most detailed scan for CNV, while the larger scans (6 mm x 6 mm, 8 mm x 8 mm, or 12 mm x 12 mm) provide a wider view useful in DR and vascular occlusion.

KNOW YOUR MACHINE AND ITS CAPABILITIES
For the clinician, it is critical to be familiar with software and capabilities of the OCTA device utilized. OCTA is a technology in evolution with continual updates. The quantitative parameters accompanying OCTA images may not be comparable when generated by different devices. The structural OCT B scan should be evaluated concurrently with the OCTA images. Use the software to scroll through the OCTA images in a layer by layer fashion if the pathology to make treatment decisions is not evident on the initial machine generated image. Consider dye-based angiography as an adjunct when needed to make treatment decisions. Expect a learning curve of 2 to 3 weeks and think in terms of flow when looking at OCTA images. RP
REFERENCES
- Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence angiography. Retina. 2015;35:2163-2180.
- Gill A, Cole ED, Novais EA, et al. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retina Vitreous. 2017;3:19.








