Intravitreal injection (IVT) is a common method of administering medication into the eye. The frequency of IVT use began to increase rapidly in the mid-2000s as steroids became available for uveitis and anti-vascular endothelial growth factor (VEGF) medications became available for wet age-related macular degeneration (AMD) and diabetic eye diseases (Figure 1). More than 2.3 million IVT procedures were performed in 2012, and conservative growth projections estimate that there might have been as many as 6 million IVT procedures in 2016.1

INTRAVITREAL INJECTIONS
The most common IVT technique is a pars plana injection, with the site of insertion usually in the inferotemporal quadrant and the needle passing through the pars plana into the mid- or posterior vitreous.2
The vitreous is 99% water and 1% macromolecular proteins, including collagen, fibrillin, and glycoproteins such as hyaluronic acid.3 Medication that is administered by pars plana IVT has been shown to spread beyond the vitreous into the aqueous humor, retina, and choroid. Pharmacokinetic studies in rabbit and pig models have demonstrated that, following pars plana IVT, concentrations of medication peak in each tissue within the first few days after injection and then gradually clear over the next several weeks. The highest levels are usually in the vitreous, with levels in the vitreous and retina still detectable up to 2 months postinjection.4,5 Medications that are commonly administered by IVT include the following:
- Steroids (eg, triamcinolone, fluocinolone, dexamethasone);
- Antibiotics (eg, vancomycin) and antivirals (eg, ganciclovir [Cytovene; Hoffmann-La Roche]);
- Anti-VEGF drugs (eg, ranibizumab [Lucentis; Genentech] and aflibercept [Eylea; Regeneron] for approved uses; bevacizumab [Avastin; Genentech], commonly used off-label for a range of retinal diseases);
- Pharmacologic vitreolytic agents (ocriplasmin [Jetrea; ThromboGenics]); and
- Ocular gene therapy products in clinical development.
From the list above, the medications that are approved (or available and used off-label) are used to treat a number of retinal diseases, including wet AMD, diabetic eye diseases such as diabetic retinopathy (DR) and diabetic macular edema (DME), retinal vein occlusion (RVO), cystoid macular edema, noninfectious uveitis, infectious endophthalmitis, and vitreomacular traction.
The primary benefit of administering a medication by IVT is that direct, targeted delivery to ocular tissues increases the bioavailability of the medication to the affected tissues and reduces systemic toxicity.2 The risks of administering a medication by IVT can be difficult to separate from the risks associated with the medication itself. Risks that have been associated with the IVT procedure include, in order of severity, ocular pain or discomfort, intraocular inflammation, cataract (if the lens is contacted during the procedure), increased intraocular pressure (particularly if injecting a large volume), bleeding (subconjunctival or vitreous), retinal tear or detachment, and infectious endophthalmitis (a rare but serious complication).6
INTRAOCULAR INFLAMMATION
Intraocular inflammation is a common, usually nonserious risk that has been associated with IVT. Multiple systems have been proposed to classify and diagnose intraocular inflammation, including some based on the site, pathology, or the course of the inflammatory response. One system, created by the Standardization of Uveitis Nomenclature (SUN) Working Group, organizes cases of intraocular inflammation into categories based on the primary site of inflammation, including the following:7
- Anterior inflammation: primary site in the anterior chamber, including iritis and anterior cyclitis;
- Intermediate inflammation: primary site in the vitreous, including pars planitis, posterior cyclitis, and hyalitis; and
- Posterior inflammation: primary site in the retina or choroid, including choroiditis, retinitis, and neuroretinitis.
There are multiple physiologic components to an inflammatory response, including the production of antibodies when a foreign pathogen is present or in cases of autoimmune activity. An inflammatory response increases the flow of blood and immune cells to the site of inflammation, dilating and increasing the permeability of the local vasculature and recruiting lymphocytes, leukocytes, and macrophages to the area.8
Increased permeability of the vasculature allows cells and proteins to leak into the aqueous humor and vitreous. The severity of an intraocular inflammatory response can therefore be evaluated by measuring the level of cells and protein in these tissues (Figure 2). Quantification of anterior cells is a clinical endpoint that measures the level of red and white blood cells in the anterior chamber, and it can be graded on a scale standardized by the SUN Working Group that ranges from 0 (no cells) to 4+ (>50 cells in a 1 mm x 1 mm slit beam field).7 Anterior flare is a clinical endpoint that measures the level of protein that accumulates in the anterior chamber, and it can be graded on a standardized (SUN) scale from 0 (no flare) to 4+ (intense flare).7
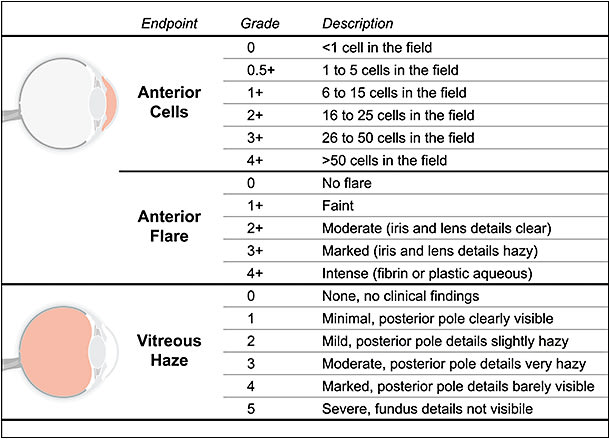
Vitreous haze is a clinical endpoint that evaluates the severity of posterior inflammation by measuring the degree to which leaked cells and protein obscure a physician’s view through the vitreous. Vitreous haze is often graded on a standardized photographic scale (according to the National Instituters of Health) from 0 (no clinical findings, clear view on fundus photography) to 5 (optic disc obscured, not visible on fundus photography).7,9,10
INFLAMMATION AFTER INTRAVITREAL INJECTION
While intraocular inflammation is a noted risk of an IVT procedure, the incidence and severity may vary considerably, depending on the medication being administered. Below is a discussion of the current evidence, when available, on the frequency, character, severity, and resolution of intraocular inflammation reported after IVT for various medications.
Anti-VEGF Drugs
Anti-VEGF IVTs are used to treat a range of retinal vascular diseases, and depending on the drug and indication, they must be administered every 4 to 8 weeks. Intraocular inflammation is among the main ocular adverse events associated with ranibizumab,11 which is approved as an IVT every 4 weeks for the following indications: (1) wet AMD; (2) macular edema following RVO; (3) DR and DME; (4) and myopic choroidal neovascularization.12 In phase 3 clinical trials of ranibizumab for wet AMD, the rate of significant (grade 3/4) intraocular inflammation after 2 years of follow-up were 2.1% (MARINA study) and 1.4% (ANCHOR study) for ranibizumab groups, compared with 0% in control groups (photodynamic therapy or sham injection, depending on the trial). The rates of mild to moderate (trace to grades 1/2) intraocular inflammation were 16.8% (MARINA) and 13.3% (ANCHOR) for ranibizumab and 12.7% (MARINA) and 3.5% in the control groups.13,14
To evaluate potential differences in the rates of intraocular inflammation after ranibizumab vs bevacizumab IVT, a large retrospective study was performed with subjects (wet AMD, DR, or RVO) who received at least a single IVT of either medication.15 The results of the study showed that there was no significant difference in the rates of anterior or posterior inflammation after ranibizumab (N=725 patients) vs bevacizumab IVT (N=1,275 patients). Anterior inflammation occurred in 1.38% of the ranibizumab IVT group (N=10) and 1.57% of the bevacizumab group (N=20), while posterior or anteroposterior inflammation occurred in 0.41% of the ranibizumab IVT group (N=3) and 0.39% of the bevacizumab IVT group (N=5).15
Large retrospective studies such as this one suggest that the rate of intraocular inflammation after bevacizumab IVT is low and similar to that with ranibizumab IVT,15 although multiple single-center studies have reported rates for bevacizumab that vary widely, from 0.3% to 14.3%, with some suggesting that this variation could be due to differences between bevacizumab lots.11
Aflibercept is approved as an IVT every 4 to 8 weeks, depending on the indication (8 weeks for wet AMD, 4 weeks for macular edema following RVO, 8 weeks for DME and DR in patients with DME).16 A review of ocular safety events from randomized, controlled trials across all indications showed an overall rate of intraocular inflammation of 2.06% for aflibercept, which was not significantly different from that for the pooled control group (2.37%).17
While these rates appear similar to what was reported for the phase 3 ranibizumab trials, comparative studies have suggested that aflibercept may actually be associated with higher rates of postinjection inflammation. A prospective, single-center, open-label study found that anterior inflammation occurred more frequently after aflibercept IVT (19%, N=10/53 patients) than ranibizumab IVT (2%, N=1/47 patients), although most cases were mild and transient, and only 1 subject (in the aflibercept group) had residual anterior inflammation beyond 7 days. Vitreous inflammation was infrequent in each group and, if present, was mild.18
A separate study compared the rate of severe inflammation after aflibercept vs ranibizumab IVT, and based on the results of a large retrospective, claims-based analysis (N=432,794 injections), it found that severe intraocular inflammation occurred more frequently after aflibercept IVT (1.06/1,000 injections), compared to ranibizumab (0.64/1,000 injections).19
A comparative case series was performed to characterize the clinical characteristics and visual outcomes of patients who had intraocular inflammation after receiving treatment with intravitreal injections of aflibercept.20 The overall incidence of intraocular inflammation was 2.25% (19 of 844 patients who received a total of 5,356 aflibercept injections). All of the cases had decreased VA at presentation, with an average decrease of 3.5 Snellen lines. Most cases had anterior-chamber inflammation, and all of them had vitritis. All of the patients were treated with topical steroids, and all but 1 regained preinjection VA an average of 33 days after injection. The remaining patient had resolution of intraocular inflammation at 28 days after injection, but the VA remained at 20/200 (from baseline 20/60), and the patient was lost to further follow-up.20
Triamcinolone Acetonide
Cases of intraocular inflammation have also been reported after the use of steroids for the treatment of retinal diseases, including macular edema. In a report of 7 patients with cystoid or diffuse macular edema who developed intraocular inflammation after a single intravitreal injection of 1 mg or 4 mg of triamcinolone acetonide, all cases were positive for anterior and posterior inflammation and had severe reductions in VA at 1 to 2 days after injection, decreasing to 20/400 and then to hand motion.21 Six of the cases resolved without treatment and returned to preinjection VA or better. One case resolved after treatment with prednisolone.21
Ocriplasmin
Ocriplasmin was engineered from human plasmin to create a pharmacologic vitreolytic agent approved as a single-use IVT for the treatment of patients with symptomatic vitreomacular adhesion (ie, vitreomacular traction).22 In 2 randomized, controlled phase 3 trials, the combined rate of intraocular inflammation at 6 months after injection was 7.1% in the ocriplasmin group (n=33/465 patients) and 3.7% in the placebo group (N=7/187 patients).23 Most cases in the ocriplasmin group and all cases in the placebo group affected the anterior chamber. Most cases resolved spontaneously. Two cases of vitritis, which were in the ocriplasmin group and deemed unrelated to study drug, did not resolve spontaneously and were treated with a steroid IVT.23
Gene Therapy
Gene therapy is an emerging medical technology that introduces a functional copy of a gene into a patient’s cells to correct an underlying genetic defect that is causing a disease (in cases of autosomal recessive or X-linked disease). By addressing the underlying cause of the disease, gene therapy has the potential to provide restorative disease-modifying effects after a single treatment.
Gene therapy uses engineered viruses, or viral vectors, to deliver genes into cells. Adeno-associated virus, or AAV, is especially well suited for use as a viral vector. AAV is a small, simple, nonenveloped virus that is straightforward to work with from a genetic engineering perspective. AAV vectors have been optimized for use in gene therapy by removing the 2 native genes and replacing them with a functional human gene.24
AAV vectors are also safe to administer. Gene delivery with AAV vectors does not alter the patient’s native DNA. AAV vectors have no viral genes remaining, reducing the possibility that a viral gene will cause an adverse event. Furthermore, while AAV has never been shown to cause disease in humans, it can elicit a weak immune response.24 Based on this immune response (whether to the AAV capsid or to the new protein being produced due to the delivered gene), some degree of intraocular inflammation may occur after the administration of AAV-based gene therapy.
Most AAV-based gene therapy product candidates are administered as a subretinal or intravitreal injection. Of the IVT gene therapy product candidates currently in clinical development, those for X-linked retinoschisis (XLRS), wet AMD, and Leber hereditary optic neuropathy (LHON) have reported data on inflammation after treatment (Table).
| RETINAL DISEASE | SPONSOR (TRIAL) | KEY FEATURES OF TRIAL | STATUS OF FINDINGS | REPORT OF INTRAOCULAR INFLAMMATION |
|---|---|---|---|---|
| XLRS | AGTC (NCT02416622) |
Phase 1/2 Multicenter Open label Dose escalation Vector: AAV2tYF-RS1 |
Preliminary results from low-dose and medium-dose groups31,32 | Majority of subjects had mild to moderate intraocular inflammation in treated eye. Inflammation generally started about 3 to 4 weeks after IVT. Inflammation generally resolved or was controlled without intervention or after treatment with topical or oral steroids. |
| Wet AMD | Genzyme (NCT01024998) |
Phase 1 Multicenter Open label Dose escalation Vector: AAV2-sFLT01 |
Published results after 52 weeks of follow-up34 | 1 subject had intraocular inflammation, from the high-dose group, moderate severity, started 1 month after IVT. Inflammation was deemed “likely to be study drug related” and resolved 5 weeks after prescription of topical steroid. |
| LHON | NEI (NCT02161380) |
Phase 1 Single center Open label Dose escalation Vector: AAV2-ND4 |
Published results from 14 subjects in low-dose and medium-dose groups37 Most subjects had follow-up through 12 months |
2 cases of asymptomatic intraocular inflammation; both cases resolved spontaneously, without intervention. |
| GenSight Biologics (NCT02064569) |
Phase 1/2 Single center Open label Dose escalation Vector: AAV2-ND4 |
Preliminary results after 48 weeks of follow-up38,39 | Intraocular inflammation and increased intraocular pressure were most common adverse events. Most cases of inflammation were mild and were treated with topical anti-inflammatory medication. 2 cases had severe inflammation and were treated with oral steroids. There were no visual or ocular sequelae related to inflammation. |
|
| Advanced RP (optogenetics) | RetroSense Therapeutics (now Allergan) (NCT02556736) |
Phase 1/2 Single center Open label Dose escalation Vector: AAV2-ChR2 |
Preliminary results from low-dose group41 | No cases of intraocular inflammation reported in the low-dose group. |
| Abbreviations: AAV, adeno-associated virus; AGTC, Applied Genetic Technologies Corporation; AMD, age-related macular degeneration; IVT, intravitreal triamcinolone acetonide; LHON, Leber hereditary optic neuropathy; NEI, National Eye Institute; RP, retinitis pigmentosa; XLRS, X-linked retinoschisis. | ||||
X-linked retinoschisis. XLRS is an inherited retinal disease characterized by schisis, or splitting of the retinal layers, and it is caused by mutations in the RS1 gene, which encodes the retinoschisin protein.25 Preclinical findings in a knockout mouse model and safety studies in rabbits and nonhuman primates supported the advancement of gene therapy product candidates to clinical trials [NCT02416622 sponsored by Applied Genetic Technologies Corporation (AGTC); NCT02317887 sponsored by the National Eye Institute].26-30 A safety study in nonhuman primates sponsored by AGTC focused on inflammation after IVT and showed that injection of an AAV vector expressing human RS1 was associated with dose-related anterior and posterior inflammation that was self-limiting and improved over time.29
Preliminary 12-month results were recently reported from the ongoing phase 1/2 clinical trial sponsored by AGTC of the safety and tolerability of an IVT of an AAV vector expressing human RS1 in patients with XLRS (NCT02416622).31,32 The trial is a multicenter, open-label, 2-stage, dose-escalation study in which the second stage will evaluate a maximum tolerated dose that is determined during the first stage. Preliminary findings from the dose-ranging stage showed that the treatment has been generally well tolerated, with mild to moderate ocular inflammation observed in the treated eye for the majority of subjects. Ocular inflammation generally occurred 3 to 4 weeks after IVT (range of 1 day to 6 months) and was either resolved or controlled after treatment with topical or oral steroids.31,32
Wet AMD. Wet AMD, typically characterized by VEGF-dependent macular edema and neovascularization, is a major cause of vision loss in older adults. The current standard of care for wet AMD is an IVT of anti-VEGF medication every 4 to 8 weeks. The goal of gene therapy for wet AMD is to provide a long-term therapeutic benefit with a single administration, which would theoretically eliminate or greatly reduce the need for frequent anti-VEGF injections.
There are multiple wet AMD gene therapy product candidates in development. One is an engineered VEGF decoy receptor, sFLT01, which is administered by IVT. A preclinical study in nonhuman primates showed that an IVT of an AAV vector expressing sFLT01 was associated with mild to moderate dose-dependent vitreal inflammation that was self-resolving.33 Based on the findings of this and other preclinical studies, Genzyme conducted a phase 1, multicenter, open-label, dose-escalation trial to evaluate the safety and tolerability of an IVT of an AAV vector expressing sFLT01 in patients with wet AMD (NCT01024998).34 One subject had moderate intraocular inflammation (iritis, vitritis, keratic precipitates) that started 1 month after treatment. The subject was from the high-dose group, and the inflammation was deemed “likely to be study drug related.” The inflammation resolved 5 weeks after prescription of difluprednate eye drops and did not recur after completion of the steroid course.34
Leber hereditary optic neuropathy. LHON is a maternally inherited disease characterized by central vision loss caused by degeneration of retinal ganglion cells, including those that form the optic nerve. Most cases of LHON are caused by mutations in any of 3 mitochondrial genes (ND1, ND4, or ND6), and more than half of cases are caused by the same mutation in ND4.35,36
Multiple clinical trials are under way to evaluate the safety, tolerability, and efficacy of an IVT of AAV vectors expressing human ND4 in patients with ND4 LHON. Initial reports from an ongoing phase 1, single-center, open-label, dose-escalation trial supported by the National Eye Institute and conducted at Bascom Palmer Eye Institute (NCT02161380) reported 2 cases of asymptomatic intraocular inflammation among 14 subjects in the low-dose and medium-dose groups. Most subjects had 12 months of follow-up, and both cases of inflammation resolved spontaneously without treatment.37
Findings have also been reported from a completed phase 1/2, single-center, open-label, dose-escalation trial (N=14) sponsored by GenSight Biologics (NCT02064569). The most common adverse events after 48 weeks of follow-up were increased intraocular pressure and intraocular inflammation (anterior and vitreous). Most cases of inflammation were mild and were treated with topical anti-inflammatory medications.
Two subjects had severe inflammation and were treated with oral steroids. Patients with nonsevere inflammation received topical steroids. There were no visual or ocular sequelae in any case related to inflammation.38 An announcement of topline results after 96 weeks of follow-up confirmed that there have not been any serious treatment-emergent adverse events and that all ocular adverse events so far have been mostly mild, well tolerated, reversible, and responsive to treatment (when needed).39
Optogenetics for advanced retinitis pigmentosa. Optogenetics is a version of gene therapy that introduces a light-sensitive protein into cells that do not normally respond to light. In many cases, the light-sensitive protein is channelrhodopsin 2 (ChR2). One of the advantages of optogenetic therapy is that it has the potential to treat patients with late-stage retinal dystrophies, even in cases in which most or all of the photoreceptors have been damaged or lost.40
A phase 1/2, single-center, open-label, dose-escalation trial is being supported by RetroSense Therapeutics (now Allergan) and conducted at the Retina Foundation of the Southwest (NCT02556736) to evaluate the safety and tolerability of an IVT of an AAV vector expressing ChR2 in patients with advanced retinitis pigmentosa. Preliminary results showed no cases of intraocular inflammation after IVT in the first cohort of subjects that received the lowest dose (N=3).41
SUMMARY
Intravitreal injection is a common procedure performed by retinal specialists. While most IVTs are routine and uncomplicated, intraocular inflammation has been associated with the procedure. The rate and severity of inflammation depend on the medication being administered. For medications administered with a single injection, such as gene therapy, most cases of inflammation seen to date have been mild, self-limiting, or responsive to treatment (when needed). RP
REFERENCES
- Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34(Suppl 12):S1-S18.
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(Suppl):S3-19.
- Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye (Lond). 2008;22:1214-1222.
- Olsen TW, Feng X, Wabner K, et al. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749-4756.
- Park SJ, Oh J, Kim YK, et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye (Lond). 2015;29:561-568.
- Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676-698.
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature (SUN) for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516.
- Rankin JA. Biological mediators of acute inflammation. AACN Clin Issues. 2004;15:3-17.
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467-471.
- Zierhut M, Deuter C, Murray PI. Classification of uveitis – current guidelines. Eur Ophthalmic Rev. 2007;77-78.
- Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56:95-113.
- Lucentis [package insert]. South San Francisco, CA: Genentech, Inc.; 2017.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432-1444.
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419-1431.
- Ladas ID, Karagiannis DA, Rouvas AA, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29:313-318.
- Eylea [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc.; 2017.
- Kitchens JW, Do DV, Boyer DS, et al. Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. 2016;123:1511-1520.
- Khanani AM, Cohen GL, Zawadzki R. A prospective masked clinical assessment of inflammation after intravitreal injection of ranibizumab or aflibercept. J Ocul Pharmacol Ther. 2016;32:216-218.
- Souied EH, Dugel PU, Ferreira A, et al. Severe ocular inflammation following ranibizumab or aflibercept injections for age-related macular degeneration: a retrospective claims database analysis. Ophthalmic Epidemiol. 2016;23:71-79.
- Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014;158:733-737.
- Roth DB, Chieh J, Spirn MJ, Green SN, Yarian DL, Chaudhry NA. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003;121:1279-1282.
- Stalmans P, Duker JS, Kaiser PK, et al. OCT-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina. 2013;33:2003-2011.
- Kaiser PK, Kampik A, Kuppermann BD, et al. Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreomacular adhesion/traction. Retina. 2015;35:1111-1127.
- Salganik M, Hirsch ML, Samulski RJ. Adeno-associated virus as a mammalian DNA vector. Microbiol Spectr. 2015;3:1-21
- Molday RS, Kellner U, Weber BH. X-linked juvenile retinoschisis: clinical diagnosis, genetic analysis, and molecular mechanisms. Prog Retin Eye Res. 2012;31:195-212.
- Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of X-linked juvenile retinoschisis. Mol Ther. 2005;12:644-651.
- Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916-926.
- Marangoni D, Wu Z, Wiley HE, et al. Preclinical safety evaluation of a recombinant AAV8 vector for X-linked retinoschisis after intravitreal administration in rabbits. Hum Gene Ther Clin Dev. 2014;25:202-211.
- Ye GJ, Budzynski E, Sonnentag P, et al. Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev. 2015;26:165-176.
- Bush RA, Zeng Y, Colosi P, et al. Preclinical dose-escalation study of intravitreal AAV-RS1 gene therapy in a mouse model of X-linked retinoschisis: dose-dependent expression and improved retinal structure and function. Hum Gene Ther. 2016;27:376-389.
- AGTC. AGTC announces topline safety data for X-linked retinoschisis phase 1/2 study; 2017. Available at: http://ir.agtc.com/releasedetail.cfm?ReleaseID=1029639 . Accessed June 28, 2017.
- Pennesi, ME, on behalf of AGTC, Biogen, and the AGTC-RS1-001 Trial Investigators. AGTC-RS1-001 phase 1/2 intravitreal gene therapy interim safety results for X-linked retinoschisis. Paper presented at: 40th Annual Macula Society Meeting; June 7-10, 2017; Singapore.
- MacLachlan TK, Lukason M, Collins M, et al. Preclinical safety evaluation of AAV2-sFLT01— a gene therapy for age-related macular degeneration. Mol Ther. 2011;19:326-334.
- Heier JS, Kherani S, Desai S, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390:50-61.
- Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57:77-86.
- Mackey DA, Oostra RJ, Rosenberg T, et al. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59:481-485.
- Guy J, Feuer WJ, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results. Ophthalmology. 2017 Jun 21. [Epub ahead of print]
- Krader CG. LHON: gene therapy for Leber’s hereditary optic neuropathy. ESCRS Euro Times Web site. http://www.eurotimes.org/lebers-hereditary-optic-neuropathy/ . Accessed November 26, 2017.
- GenSight Biologics. GenSight Biologics reports long-term positive safety and visual acuity results at week 96 in phase I/II study of GS010 for the treatment of Leber’s hereditary optic neuropathy (LHON); 2017. Available at http://www.gensight-biologics.com/uploads/Modules/InvestorNews/gensight-biologics---pr---96-weeks-results_vdef.pdf . Accessed June 28, 2017.
- Klapper SD, Swiersy A, Bamberg E, Busskamp V. Biophysical properties of optogenetic tools and their application for vision restoration approaches. Front Syst Neurosci. 2016;10:74.
- RetroSense Therapeutics. RetroSense Therapeutics completes low dose cohort in clinical trial of novel gene therapy application of optogenetics; 2016. Available at: http://www.businesswire.com/news/home/20160810005731/en . Accessed June 28, 2017.








