Wet AMD is a complex disease with multiple pathogenic mechanisms. The current standard of care, anti-vascular endothelial growth factor (anti-VEGF), has markedly improved the management of these patients and has established a new efficacy paradigm of vision stabilization and even vision improvement in many patients. However, anti-VEGF treatments require frequent and long-term administration to maintain vision gains, and some patients may lose their initial vision gains over time, in part because of recurrent leakage from the CNV complex. Alternative therapies that improve outcomes compared with the current standard of care are needed. This article reviews drugs in development toward that end.
VEGF INHIBITORS
Anti-VEGF therapy has revolutionized care for patients with AMD, but treatment burden remains a challenge for patients and retina specialists. New drugs that target different proteins could produce better visual results with fewer treatments (Table 1).
| Brolucizumab (RTH258, ESBA1008) | Abicipar pegol (AGN-150998, MP0112) | OPT-302 (soluble VEGFR-3; VGX 300) | |
|---|---|---|---|
| Developer | Novartis | Molecular Partners and Allergan | Opthea |
| Route | Intravitreal | Intravitreal | Intravitreal |
| Dose | 3.0-6.0 mg q4w to q12w | 2 mg q8w to q12w | 0.5-2.0 mg |
| Clinical trial status | Phase 3 | Phase 3 | Phase 2 |
Brolucizumab (RTH258, ESBA1008)
Brolucizumab (Novartis) is a humanized single chain antibody fragment that has high affinity to all isoforms of VEGF-A. It has a molecular weight of 26 kDa (less than aflibercept [Eylea; Regeneron] and ranibizumab [Lucentis; Genentech]), which makes it possible to prepare higher molar concentrations and administer up to 6 mg of the drug in a single 0.05 mL intravitreal injection, resulting in longer duration of effect, rapid systemic clearance, and better ocular tissue penetration. Brolucizumab is in phase 3 trials (HAWK and HARRIER) and the latest results show that brolucizumab is noninferior to aflibercept.1
The HAWK and HARRIER trials are multicenter, randomized, double-masked trials of brolucizumab (6 mg) vs aflibercept (3 mg). The 2 trials comprised more than 1,800 wet AMD patients. All patients received 3 monthly loading doses of the assigned treatment, followed by 12-week dosing intervals for brolucizumab eyes, with an option to adjust to an 8-week dosing based on disease activity. Aflibercept was administered every 8 weeks after the loading phase.
At 48 weeks, brolucizumab achieved robust and consistent visual gains, and more than half of the study eyes were maintained on a 12-week dosing interval. Other outcomes that favored brolucizumab included the following:
- Significantly less disease activity at week 16,
- Superior central subfield thickness improvement at weeks 16 and 48,
- Significantly fewer patients with presence of intraretinal and/or subretinal fluid, and
- Overall safety comparable to aflibercept.
Both trials will continue to follow patients through 96 weeks (NCT02307682 and NCT02434328).
Abicipar Pegol (AGN-150998, MP0112)
Abicipar pegol (Molecular Partners and Allergan) is an anti-VEGF-A designed ankyrin repeat protein (DARPin), which is a genetically modified antibody mimetic protein that is highly specific and has high-affinity target protein binding properties. Abicipar is an antagonist of VEGF-A that inhibits all relevant subtypes of VEGF-A with very high potency.
DARPins are recombinant proteins genetically engineered to bind to a specific target. Besides being small, potent, and having high specificity and binding affinity, DARPins are stable, which translates into good shelf-life, and highly soluble, which could allow for mixing with other agents and formulation in novel drug delivery systems (Figure 1).
During the Phase II REACH study, 25 patients were randomized to abicipar 1 mg, 23 patients to abicipar 2 mg, and 16 patients to ranibizumab 0.5 mg. Results from this study showed an improvement of mean visual acuity from baseline of 9 letters for abicipar 2 mg and 7.1 letters for abicipar 1 mg compared to 4.7 letters for ranibizumab 0.5 mg. Although this study was not powered to show statistical significance, it provided promising results that paved the way for a Phase III program consisting of 2 trials: CDER and SEQUOIA.
The CDER and SEQUOIA trials are ongoing phase 3 trials that consist of two 96-week trials. Recruitment is ongoing in the trials that have a planned enrollment of about 900 patients each. Eligible patients are being randomized to 3 groups to receive abicipar 2 mg at weeks 0, 4, and 8 followed by injections every 8 weeks; abicipar 2 mg injections at week 0, 4, and 12 followed by injections every 12 weeks; or ranibizumab 0.5 mg every 4 weeks. The primary endpoint is the proportion of patients with stable vision (≤15 letters change from baseline BCVA). Secondary endpoints are change from baseline in Early Treatment Diabetic Retinopathy Study (ETDRS) BCVA, change from baseline in central retinal thickness, percentage of patients with a BCVA gain ≥15 letters, and change from baseline in the National Eye Institute Visual Functioning Questionnaire-25 composite score (NCT02462928 and NCT02462486).
OPT-302 (soluble VEGFR-3; VGX 300)
OPT-302 (Opthea) is a soluble form of VEGF receptor 3 comprising the extracellular domains 1-3 of human VEGF receptor (VEGFR-3) and the Fc fragment of human IgG1. The VEGFR-3 or “trap” molecule blocks the activity of the proteins VEGF-C and VEGF-D, which cause blood vessels to grow and leak and contribute to the pathophysiology of retinal diseases (Figure 2).
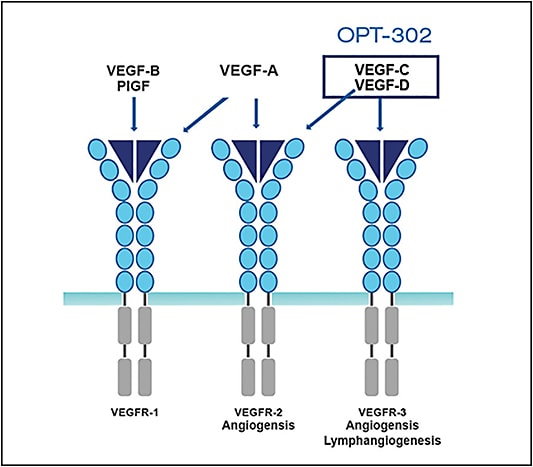
OPT-302 is used in combination with inhibitors of VEGF-A (eg, ranibizumab or aflibercept). Combination therapy of OPT-302 and a VEGF-A inhibitor achieves more complete blockade of members of the VEGF family. A phase 1/2a dose-escalation study demonstrated safety and tolerability of OPT-302 as monotherapy and in combination with ranibizumab. The combination group had better visual results and previous poor responders to anti-VEGF-A therapy fared better with the addition of OPT-302. A phase 2 multicenter, randomized, dose-ranging study is under way, investigating 2 doses of OPT-302 in combination with ranibizumab compared with ranibizumab with sham, over 6 consecutive monthly dosing cycles in participants with neovascular AMD (NCT03345082).
Rabinizumab Port Delivery System
The ranibizumab port delivery system (RPDS; Genentech) is a refillable reservoir system designed to gradually release ranibizumab (Figure 3). The drug is released using a diffusion-control mechanism and the port is placed under the conjunctiva, fixed to the pars plana, and no sutures are needed. The port is then refilled as an in-office procedure with the help of a refill needle system that simultaneously introduces the drug into the reservoir and removes any remaining contents.

Currently the drug is being investigated in a phase 2 trial (LADDER; Long Acting Delivery of Ranibizumab). The LADDER study is a multicenter, randomized, active treatment-controlled clinical trial investigating the efficacy and safety of the RPDS for the sustained delivery of ranibizumab in participants with wet AMD who have previously responded to treatment with anti-VEGF therapies. The trial is evaluating the implant with 3 different ranibizumab formulations. The primary endpoint is the time until a patient first requires the RPDS implant to be refilled according to protocol-defined refill criteria (NCT02510794). This trial will help to determine whether the RPDS implant can reduce the treatment burden for people with wet AMD, including fewer office visits and retreatments, while achieving vision gains comparable to patients treated with monthly injections.
Aflibercept
In December 2017, the US Food and Drug Administration (FDA) accepted for review a supplemental Biologics License Application (sBLA) by Regeneron for a 12-week dosing interval of aflibercept injection in patients with wet AMD. Under the Prescription Drug User Fee Act (PDUFA), the goal for a standard review of an sBLA is 10 months from submission, for a target action date of August 11, 2018.2
ANTI-PLATELET-DERIVED GROWTH FACTOR
Platelet-derived growth factor (PDGF) binds to a tyrosine kinase receptor on pericytes, which is critical for survival, recruitment, and maturation of pericytes. Inhibiting PDGF to strip pericytes from the neovacular complexes might be a useful approach to improve long-term visual outcomes in patients with wet AMD, as it might leave the underlying endothelial cells more sensitive to the effects of VEGF blockade.
Several anti-PDGF molecules have failed in clinical trials. One of these was pegpleranib (Fovista, E-10030; Ophthotech), a pegylated DNA aptamer that selectively binds to PDGF-BB and PDGF-AB, thereby disrupting the interaction with their tyrosine kinase receptors in the pericytes and leading to pericyte stripping from the underlying neovascular complex. A Phase 2b trial achieved primary endpoint superiority (mean change in visual acuity at 24 weeks) with statistical significance in patients receiving the combination of pegpleranib (1.5 mg) and ranibizumab.
In the phase 3 OPH1004 trial, subjects receiving pegpleranib in combination with aflibercept or bevacizumab (Avastin; Genentech) gained a mean of 9.42 letters of vision on the ETDRS standardized chart at 12 months, compared to a mean gain of 9.04 ETDRS letters in patients receiving aflibercept or bevacizumab monotherapy, resulting in a difference of 0.38 ETDRS letters (P=.74). These results were not statistically significant. In addition, there was no clinically meaningful visual benefit in the prespecified secondary endpoints when pegpleranib was added to the aflibercept or bevacizumab regimens. Based on these data, the company decided to stop treating patients in the second year of the OPH1004 study (NCT01940887). In the 2 pivotal phase 3 clinical trials (OPH1002 and OPH1003) investigating the superiority of pegpleranib in combination with ranibizumab compared to ranibizumab monotherapy for the treatment of wet AMD, the addition of pegpleranib to a monthly ranibizumab regimen did not result in benefit as measured by the mean change in visual acuity at 12 months (NCT01944839 and NCT01940900).
Another anti-PDGF molecule that did not show benenfit in clinical trials was rinucumab (REGN2176-3; Regeneron), a monoclonal anti-PDGF receptor beta antibody, formulated with aflibercept in a single injection. The phase 2 CAPELLA study evaluating aflibercept coformulated with rinucumab in patients with wet AMD showed that the combination therapy did not demonstrate an improvement in best corrected visual acuity (BCVA) compared to intravitreal aflibercept injection monotherapy at 12 weeks, the primary endpoint of the study (NCT02418754).
Another anti-PDGF molecule that did not progress through clinical trials is DE-120 (Santen), a dual VEGF and PDGF inhibitor, which was being studied in a phase 2 trial to evaluate the safety and efficacy of the intravitreal drug as monotherapy and along with a single injection of aflibercept in subjects with treatment-naïve active subfoveal CNV secondary to AMD. The company discontinued development in 2017.3
Vorolanib (X-82, CM-082)
Vorolanib (Tyrogenex) is a tyrosine kinase inhibitor derived from the anti-cancer agent sunitinib that blocks the kinase activity of all receptor subtypes for VEGF and PDGF. It is an oral agent being developed to inhibit VEGFR and platelet-derived growth factor receptor (PDGFR). The ability of this drug to bind to these receptors is expected to be effective in angiogenic diseases such as wet AMD. The APEX study is a phase 2 study of vorolanib plus as‐needed intravitreal anti‐VEGF compared to as‐needed intravitreal anti‐VEGF alone in patients with wet AMD. Subjects are being treated for a total of 52 weeks with 1 of 3 doses of vorolanib or placebo (NCT02348359).
ANGIOPOETIN INHIBITORS
Angiopoietin 1 and 2 are of key importance in the homeostasis of the vascular compartment. angiopoietin 1 is a strong agonist that stimulates phosphorylation of the Tie2 receptor and angiopoietin 2 is an antagonist that competes with angiopoietin 1 and inhibits Tie2 phosphorylation.4-5 Angiopoietin 1 stabilizes mature vasculature by promoting recruitment of pericytes and smooth muscle cells and suppressing vascular leakage.6 High expression of angiopoietin 1 in the retina strongly suppresses VEGF-induced neovascularization and leakage.6-8 Through inhibition of Tie2, angiopoietin 2 destabilizes the endothelial cell layer, leading to fluid leakage. Angiopoietin 2 also renders the endothelial cell layer more responsive to VEGF and other proangiogenic factors.9 Not surprisingly, angiopoietin 2 is increased in proangiogenic diseases, including retinal vascular diseases10-11 such as pathologic retinal neovascularization or CNV.12-15 Therefore, selective neutralization of both VEGF and angiopoietin 2 may further normalize the pathologic ocular vasculature compared to anti-VEGF monotherapy.11
Nesvacumab (REGN910-3)
Nesvacumab (Regeneron) is a human IgG1 monoclonal antibody directed against angiopoietin 2 that blocks its interaction with the Tie2 receptor. A phase 1 study (NCT01997164) showed no significant adverse effects and good functional and anatomical outcomes. Phase 2 trials for wet AMD (ONYX; NCT02713204) using nesvacumab combined with aflibercept are under way and results are awaited.
RG7716 (RO6867461)
RG7716 (Roche) is a bispecific human IgG1 monoclonal antibody that binds to both angiopoietin 2 and VEGF-A. A Phase 1 trial (NCT01941082) in patients with persistent CNV activity despite 3 anti-VEGF injections showed that the drug was well tolerated, and improvements in BCVA and OCT parameters were observed. A phase 2 safety, tolerability, pharmacokinetics, and efficacy study (AVENUE; NCT02484690) in patients with CNV secondary to AMD is under way and results are awaited.
The STAIRWAY trial (NCT03038880) is a phase 2, multicenter, randomized, active comparator-controlled, 52-week study. The study is investigating the efficacy, safety, and pharmacokinetics of RG7716 administered with extended dosing regimens in treatment-naive participants with wet AMD. The study contains 3 arms. In the first, RO6867461 will be given intravitreally at short interval duration. In the second arm, RO6867461 will be given intravitreally at long interval duration. In the sham arm, ranibizumab will be given intravitreally. The primary outcome measure is change from baseline in BCVA at week 40. Results are awaited.
Roche recently announced encouraging results in DME from the BOULEVARD study.16 The trial met its primary endpoint, with RG7716 demonstrating statistically significant BCVA gains over ranibizumab at week 24 in anti-VEGF treatment-naïve patients with DME. When factored into a linear model adjusting for baseline BCVA and randomization factors, the 6.0-mg RG7716 group gained a mean of 13.9 letters from baseline, which was an improvement of 3.6 letters over the 10.3 letters gained by the 0.3-mg ranibizumab group (P=.03). All AMD studies have finished enrollment and are in follow-up. With the encouraging DME results, investigators are looking forward to results for RG7716 in AMD.
OTHER MOLECULAR TARGETS
AKB-9778 and ARP-1536 (Aerpio) are inhibitors of the vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) enzyme. Both molecules are in preclinical stage. AKB-9778 binds to and inhibits the intracellular catalytic domain of VE-PTP that inactivates Tie2. Inhibition of VE-PTP has shown the ability to activate the Tie2 receptor irrespective of extracellular levels of its binding ligands, angiopoietin 1 or angiopoietin-2, and could be an efficient pharmacologic approach to restoring Tie2 activation. ARP-1536 activates the Tie2 receptor in a dose-dependent manner with a preclinical efficacy profile similar to AKB-9778. The company plans on developing ARP-1536 in combination with anti-VEGF therapy for the treatment of wet AMD and DME, with the goal of monthly or quarterly dosing.
Studies have shown overexpression of tissue factor in pathologic neovascularization.17 ICON-1 (hI-con1; Iconic Therapeutics) is a chimeric protein that inhibits tissue factor. The phase 2 EMERGE study of ICON-1 evaluated its biological effect on CNV. Choroidal neovascularization was reduced at 6 months in patients with AMD who were treated with ICON-1 (NCT02358889).
Carotuximab (TRC105; Tracon Pharmaceuticals, formulated for ophthalmic use as DE-122 by Santen) is an inhibitor of endoglin/CD105, which is essential to angiogenesis. Santen presented encouraging data in combating refractory wet AMD in a 12-patient, phase 1/2 clinical trial for carotuximab (NCT02555306) at the Angiogenesis 2018 conference in Miami, Florida. The drug, delivered via intravitreal injection at 4 different dose levels (3 patients at each dose), was reported to be well tolerated while displaying positive biological activity as measured by mean change in central subfield thickness. The drug is now in a phase 2a trial in combination with ranibizumab (NCT03211234).
CONCLUSION
Extensive research is being carried out throughout the world to develop better options to manage wet AMD. At present, multiple intravitreal injection therapy is the only proven option in terms of vision gain. However, existing molecules have primarily targeted VEGF, which does not seem to be enough. We have highlighted some of the promising drugs being explored in 2018 that may help us manage wet AMD better in the future. RP
REFERENCES
- Dugel P, et al. HAWK & HARRIER: 48-week results of 2 multi-centered, randomized, double-masked trials of brolucizumab versus aflibercept for neovascular AMD. Presented at: The American Academy of Ophthalmology 2017 Annual Meeting on November 10, 2017, New Orleans.
- Regeneron announces FDA acceptance of SBLA filing for 12-week dosing of Eylea (aflibercept) injection for patients with wet AMD. https://www.prnewswire.com/news-releases/regeneron-announces-fda-acceptance-of-sbla-filing-for-12-week-dosing-of-eylea-aflibercept-injection-for-patients-with-wet-amd-300569395.html . Accessed March 21, 2018.
- Santen. Research & development. http://www.santen.com/en/rd/pdf/pipeline.pdf . Accessed March 21, 2018.
- Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55-60.
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14(1):25-36.
- Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11(10):865-873.
- Nambu H, Umeda N, Kachi S, et al. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol. 2005;204(1):227-235.
- Benest AV, Kruse K, Savant S, et al. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013;8(8):e70459.
- Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. J Invest Ophthalmol Vis Sci. 1999;40(9):1912-1920.
- Regula JT, Lundh von Leithner P, Foxton RT, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016;8(11):1265-1288.
- Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3(3):411-423.
- Hackett SF, Ozaki H, Strauss RW, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184(3):275-284.
- Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192(2):182-187.
- Oshima Y, Oshima S, Nambu H, et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19(8):963-965.
- Roche. Investor update. https://www.roche.com/investors/updates/inv-update-2018-02-12.htm . Accessed March 7, 2018.
- Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125 Suppl 2:S36-S38.








