Diabetic retinopathy (DR) is the leading cause of blindness in adults in the United States.1 A study by the US Centers for Disease Control and Prevention projected that 48 million people in the United States will have diabetes in 2050.2 The number of people with DR is expected to increase to approximately 16 million in 2050.3 Diabetic macular edema (DME) is the leading cause of visual loss in patients with DR.4
DEFINITIONS
The Early Treatment Diabetic Retinopathy Study (ETDRS) defines clinically significant macular edema (CSME) as thickening of the retina at or within 500 µm of the center of the macula, hard exudates at or within 500 µm of the center of the macula with adjacent retinal thickening, and retinal thickening of 1 disc diameter or larger within 1 disc diameter of the center of the macula. More recently, the terms focal and diffuse have been used to classify 2 different types of DME. Diagnostic tools that help differentiate the 2 types include fundus biomicroscopy, fluorescein angiography (FA), and OCT. Focal DME is characterized by localized leakage from microaneurysms (MA) (Figure 1). In diffuse DME, there is no definable demarcation around the areas of leakage, and the leakage involves the entire circumference of the fovea. Diabetic macular edema can also be categorized as center-involving DME, in which the central macula is affected, or non-center-involving DME. This review of therapeutic options for DME focuses on treatment options for focal and diffuse DME.

LASER TREATMENT OPTIONS
Laser Photocoagulation
Focal and/or grid laser photocoagulation was the standard treatment for DME prior to the arrival of intravitreal anti-VEGF and corticosteroid agents. ETDRS demonstrated the effectiveness of focal/grid laser photocoagulation in reducing visual loss from CSME and established it as the mainstay of treatment.5 The mechanism by which laser photocoagulation reduces DME is unclear for diffuse DME. Investigators have suggested that biochemical changes within the RPE results in decreased leakage and edema or increased function of the RPE pump mechanism, particularly for micropulse laser.6 Also, incidental direct photocoagulation of retinal MA or leaky capillaries may occur in grid laser photocoagulation in diffuse DME.
Another hypothesis involves increasing oxygenation to the inner retina. Laser photocoagulation leads to destruction of the outer retina and RPE, which improves diffusion of oxygen from the choriocapillaris to the inner retina. Laser scars may also decrease local production of VEGF by ischemic retina, leading to a decrease in DME. The results of ETDRS demonstrated that laser treatment prevents or reverses visual loss from CSME. In the study, reduction of VA by 2 lines occurred in 24% of untreated eyes and 12% in eyes that received laser photocoagulation.5 An updated ETDRS guideline recommended focal/grid laser photocoagulation as the first-line therapy for non-center-involving CSME.7 The standard setting for focal/grid laser photocoagulation is 50 µm to 100 µm, 0.05 second to 0.1 second duration, and more than 1 burn width apart.
Focal vs Grid Laser Photocoagulation
According to the modified ETDRS protocol, focal laser photocoagulation is used to treated non-center-involving focal CSME. Laser treatment is applied to areas of discrete leaking MA between 500 µm and 3,000 µm from the fovea. However, treatment may be applied between 300 µm and 500 µm of the fovea if the central-involving CSME persists after initial focal photocoagulation and if VA is worse than 20/40. Focal laser can greatly benefit focal CSME that exhibit hard exudates clustered in a ring or circinate pattern around the leaking MA. On the contrary, grid laser photocoagulation is used to treat diffuse CSME. The laser treatment can be applied to areas of diffuse leakage more than 500 µm from the fovea and more than 500 µm from the temporal margin of the optic disease.8
In many clinical trials, focal laser photocoagulation is used to treat under the microaneurysm to create whitening of the RPE. We do not believe this to be an effective treatment of the leaking microaneurysm. When performing focal/grid laser photocoagulation in our office, we pull the focus back from the RPE and onto the retinal surface to directly create whitening of the microaneurysm instead of the underlying RPE. We believe this to be a more effective method of treating microaneurysms with laser photocoagulation.
Complications of Laser Treatment
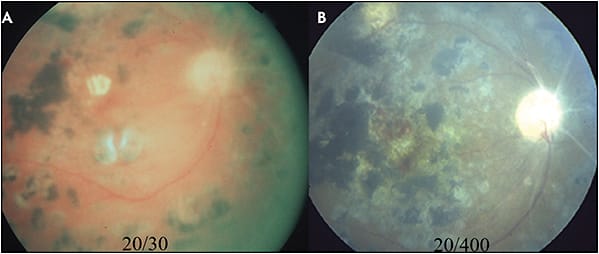
Focal/grid laser photocoagulation can produce undesirable central scotoma and loss of central vision due to the laser scars because of destruction of the RPE and surrounding retinal tissue. The laser scars can enlarge beyond the original laser spot size over time, which is of particular concern for patients receiving laser treatments close to the fovea. The expansion of the laser scar can encroach upon and threaten central vision (Figure 2).9-10 Additionally, the use of small-size, short-duration, intense laser can lead to destruction of Bruch’s membrane and formation of a choroidal neovascularization (CNV) (Figure 3).

Subthreshold Diode Micropulse Laser Therapy
Conventional focal/grid laser photocoagulation produces visible retinal tissue reaction, indicating direct damage to the retina. An alternative technique, known as subthreshold diode micropulse (SDM) laser therapy, produces less retinal damage. It delivers longer wavelength (577 nm and 810 nm) lasers at shorter pulses of 100 ms to 300 ms in duration. The shorter and less intense laser exposure selectively targets the RPE and spares the neurosensory retina.11 The low energy also confines the thermal effect of the laser and reduces spread to the surrounding tissue. This reduces the vision-threatening complications associated with laser therapy including scarring and scotoma.
A recent meta-analysis of randomized controlled trials comparing the outcomes of SDM and conventional laser treatment in DME confirmed similar VA outcome and central macular thickness after treatment for both laser treatments.12 The effectiveness of SDM combined with an attractive safety profile provides vitreoretinal surgeons with another option to treat DME.
PHARMACOTHERAPEUTIC TREATMENT
Laser therapy is being rapidly replaced by pharmacotherapeutic treatment with the advent and approval of intravitreal agents, which can be used to treat focal and diffuse DME.
Intravitreal Anti-VEGF Agents
Anti-VEGF agents are the first-line treatment options for many vitreoretinal specialists in treating DME. These agents have supplanted laser photocoagulation as the treatment of choice for DME, particularly for diffuse DME.
Ranibizumab (Lucentis; Genentech) is a monoclonal antibody fragment that inhibits VEGF-A. Bevacizumab (Avastin; Genentech) is a full-length antibody against VEGF-A. A newer agent, aflibercept (Eylea; Regeneron), is a fusion recombinant protein that inhibits all isoforms of VEGF-A, VEGF-B, and placental growth factors. Of these 3 agents, only ranibizumab and aflibercept are approved by the US Food and Drug Administration (FDA) for DME treatment.
Ranibizumab
Ranibizumab has been shown to be effective in multiple randomized clinical trials. In RISE, patients were randomized to monthly ranibizumab injections vs sham injections. At 2 years, significantly more patients in the ranibizumab group gained ≥15 letters. In the RIDE study, patients were randomized to the same treatment options. This study similarly showed that patients who received ranibizumab had more significant gain in VA when compared to sham injections. Patients who received ranibizumab also had decreased worsening of DR and received fewer laser treatments.13 In READ-2, the efficacy of ranibizumab alone, focal/grid laser treatment alone, and the combination of ranibizumab and focal/grid laser treatment were compared. At the primary 6-month endpoint, treatment of ranibizumab alone was superior compared with laser treatment alone and combination therapy. From 6 months to 24 months, as-needed treatment with ranibizumab maintained VA.14
Bevacizumab
Although bevacizumab is not FDA approved and is considered “off-label” for DME, it is widely used by vitreoretinal specialists to treat DME, and many even choose bevacizumab as the initial intravitreal agent. In BOLT, a randomized controlled clinical trial, researchers compared bevacizumab monotherapy to laser monotherapy. At 12 months, the bevacizumab group had a greater improvement in VA than the laser group. The central macular thickness also decreased more significantly in the bevacizumab group compared to the laser group.15
Aflibercept
Aflibercept is the newest anti-VEGF agent approved for treatment of DME. The DA VINCI randomized clinical trial compared aflibercept monotherapy to laser monotherapy. At 52 weeks, the aflibercept groups had superior improvement in VA compared to the laser monotherapy group. There was also a more significant decrease in central macular thickness in the aflibercept group.16
Which Anti-VEGF Agent for Treatment Initiation?
Prior to DCRC.net Protocol T, retina specialists had no guidance for anti-VEGF agent selection. Bevacizumab is a reasonable choice for treatment initiation because it is the most cost effective. Many retina specialists switch to another anti-VEGF or corticosteroid agent when bevacizumab does not produce a desirable decrease in DME.
The results of protocol T provide an evidence-based guideline for anti-VEGF selection. It is a randomized clinical trial that evaluated functional and structural outcome of eyes with DME treated with ranibizumab, bevacizumab, or aflibercept. The results showed that in eyes with better baseline VA (20/32 to 20/40), the difference in mean improvement in letters among the 3 anti-VEGF agents was not statistically significant. In eyes with worse baseline VA (20/50 to 20/320), the mean improvement for aflibercept, bevacizumab, and ranibizumab was, respectively, 18.9, 11.8, and 14.2 letters, at 1 year and 18.3, 13.3, and 16.1 letters at 2 years. Aflibercept’s superiority compared to bevacizumab and ranibizumab was statistically significant in eyes with worse baseline VA. The average number of injections was comparable in all groups. Central subfield thickness also improved in all groups.17-18
It is reasonable to begin treatment with bevacizumab due to its efficacy and low cost because there was no statistically significant difference among the 3 agents in Protocol T. However, in eyes with worse baseline VA, it may be more appropriate to start with aflibercept, according to the results.
Intravitreal Corticosteroid Agents
Intravitreal corticosteroid agents are increasingly used for treatment of recalcitrant DME that has failed laser photocoagulation and intravitreal anti-VEGF agents. Several corticosteroid agents are available, including triamcinolone acetonide (Kenalog from Bristol-Myers Squibb, Triesence from Alcon, and Trivaris from Allergan), dexamethasone (Ozurdex; Allergan), and fluocinolone acetonide (Iluvien; Alimera Sciences). Among these agents, only dexamethasone and fluocinolone are FDA approved for DME treatment. These corticosteroids provide an alternative option for patients with persistent DME. However, they are associated with increased risk of cataract-related vision loss and elevated intraocular pressure (IOP). They should be used with caution in phakic patients or glaucoma suspects and patients with glaucoma.
Triamcinolone Acetonide
In a randomized clinical trial by DRCR.net , patients were randomized into 3 different treatment groups: 1-mg preservative-free intravitreal triamcinolone, 4-mg triamcinolone, and focal/grid laser photocoagulation for the treatment of DME. In all treatment groups, more eyes had improvement in VA than worsening. Additionally, intravitreal triamcinolone was also associated with high risks of cataract progression and elevated IOP. This study did not demonstrate a long-term benefit of intravitreal triamcinolone.19
Dexamethasone Intravitreal Implant
Dexamethasone is available as a 0.7-mg intravitreal implant that can be used in pseudophakic and phakic patients with DME. In pseudophakic patients, the intraocular lens implant must be in the posterior chamber to decrease the risk of the implant migrating into the anterior chamber. In MEAD, patients were randomized to 0.7-mg dexamethasone, 0.35-mg dexamethasone, and sham treatment. A greater percentage of patients in the dexamethasone groups had ≥15-letter improvement from baseline compared to the sham group. The average reduction in central macular thickness was also greater in the dexamethasone groups. Patients in the dexamethasone groups had significantly increased risk of cataract progression and elevated IOP.20 Figure 4 shows a patient with persistent DME and the response to dexamethasone injection.

Sustained-Release Fluocinolone Acetonide Implant
The sustained-release fluocinolone acetonide 0.19-mg implant is designed to continuously release a low dose of medication for 36 months. It is approved by the FDA for the treatment of DME in patients who did not experience elevated IOP with previous corticosteroid treatment. The FAME study consisted for 3 study groups: 0.2 µg fluocinolone acetonide, 0.5 µg fluocinolone acetonide, and sham treatment. Results of the study showed that patients who received fluocinolone had a significant improvement in VA compared to the sham group. However, the fluocinolone group also had a significant risk of elevated IOP, with a subgroup of patients requiring IOP-lowering medications and surgeries.21
Which Monotherapy Should Be Used?
Because of the number of choices available, it may be difficult to decide which intravitreal agent is best for a specific patient. Intravitreal anti-VEGF tends to be the first-line treatment in our practice. Anti-VEGF is a better choice for DME in patients with PDR who are at high risk for IOP spike, such as patients with glaucoma or patients who are known steroid responders. Anti-VEGF is also preferred in young patients who are phakic because intravitreal steroids induce cataract growth, which will decrease the patient’s accommodative ability and quality of vision.
When the DME is refractory to 1 anti-VEGF agent after at least 3 injections, one may switch the patient to a different anti-VEGF agent or add dexamethasone. As stated above, dexamethasone has undesirable complications that can be avoided if a different anti-VEGF is effective in treating the DME. However, dexamethasone may be preferred in DME patients who are pseudophakic and have low risk of IOP issues because it is an effective agent and reduces the number of injections a patient receives. Dexamethasone may also be a better option in patients who have had a preceding inflammatory event, such as recent cataract extraction or other intraocular surgeries.
Laser in Combination With Intravitreal Agents
Several studies have evaluated the efficacy in combining laser treatment with intravitreal agents. In RESTORE, ranibizumab monotherapy, laser monotherapy, and combined ranibizumab and laser therapy were compared. The study found that ranibizumab alone or combined with laser therapy were superior to laser monotherapy in improving VA. The central macular thickness was also significantly reduced with ranibizumab monotherapy and combined therapy vs laser monotherapy.
The DCRC.net Protocol I study compared 4 different treatment groups: sham injection plus prompt laser, ranibizumab 0.5 mg plus prompt laser, ranibizumab 0.5 mg plus deferred laser, and triamcinolone 4 mg plus prompt laser in patients with DME. At 1 year, ranibizumab plus prompt or deferred laser achieved superior results in VA. The ranibizumab plus deferred laser had the best VA outcome of all the groups. However, the ranibizumab plus prompt laser group had similar VA outcome and lower number of injections.22
As we know, DME is a multifactorial disease and may require multiple treatment agents to achieve adequate results. In many of the above clinical trials, many patients who received intravitreal anti-VEGF agents also required focal/grid laser photocoagulation during the duration of the studies. While anti-VEGF agents have largely supplanted laser photocoagulation as the first-line treatment for DME, there is still a role for laser photocoagulation especially when used as an adjunct treatment to anti-VEGF agents to achieve synergistic results.
In our practice, we often use a combination approach with anti-VEGF and focal/grid laser photocoagulation. Focal/grid laser photocoagulation is particularly effective with focal leakage from a microaneurysm and a circinate pattern of hard exudates surrounding that microaneurysm. Combining anti-VEGF and focal/grid laser photocoagulation may produce a longer lasting effect and reduce the number of anti-VEGF injections a patient receives.
Intravitreal Anti-VEGF Combined With Corticosteroid Implants
Another option that we occasionally employ is combining intravitreal anti-VEGF with dexamethasone. This combination is typically used in patients whose DME worsened after an inflammatory event such as cataract extraction or PRP, and the DME can longer be satisfactorily controlled with anti-VEGF alone. Because the DME may have been exacerbated by the inflammation, introducing dexamethasone may help reduce the inflammation and gain better control the DME. Of course, we must again keep in mind the complications of dexamethasone and choose patients appropriately.
SUMMARY
After ETDRS published their results of focal/grid laser photocoagulation in DME, laser treatment became the gold standard for treatment of DME and remained so for many decades. Focal laser photocoagulation reduces vision loss in patients with non-centered focal DME, and grid laser photocoagulation for patients with diffuse DME. However, anti-VEGF agents have supplanted laser treatment as the first-line treatment for DME in both focal and diffuse DME. As discussed above, focal/grid laser photocoagulation may still have an important role as adjuvant therapy, because studies have demonstrated a synergistic effect when used in combination with intravitreal agents either as prompt or deferred treatment. As second-line treatment for resistant DME, intravitreal corticosteroid agents have shown to be effective in DME. RP
REFERENCES
- Fong DS, Aiello L, Gardner TW, et al; American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84-S87.
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence of future diabetes burden: U.S., 2005-2020. Diabetes Care. 2006;29(9):2114-2116.
- Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126(12):1740-1747.
- Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346-354.
- Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12);1796-1806.
- Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001;132(3):427-429.
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987;94(7):761-764.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network, Fong DS, Strauber SF, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469-480.
- Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109(11):1549-1551.
- Shah SS, Schachat AP, Murphy RP, Fine SL. The evolution of argon laser photocoagulation scars in patients with the ocular histoplasmosis syndrome. Arch Ophthalmol. 1988;106(11):1533-1536.
- Yu AK, Merrill KD, Truong SN, Forward KM, Morse LS, Telander DG. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Opththalmol Vis Sci. 2013;54(3):2216-2224.
- Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: A meta-analysis of randomized controlled trials. Retina. 2016;36(11):2059-2065.
- Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022.
- Nguyen QD, Shah SM, Khwaja AA, et al; READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146-2151.
- Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078-1086.
- Do DV, Nguyen QD, Boyer D, et al; da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF trap-eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658-1665.
- Wells JA, Glassman AR, Ayala AR, et al; Diabetic Retinopathy Cliical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203.
- Wells JA, Glassman AR, Ayala AR, et al. Diabetic Retinopathy Cliical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- Diabetic Retinopathy Clinical Research Network (DCRC.net ), Beck RW, Edwards AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127(3):245-251.
- Boyer DS, Yoon YH, Belfort R Jr., et al; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
- Campochiaro PA, Brown DM, Pearson A, et al; FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125-2132.
- Elman MJ, Avala A, Bressler NM, et al; Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381.








