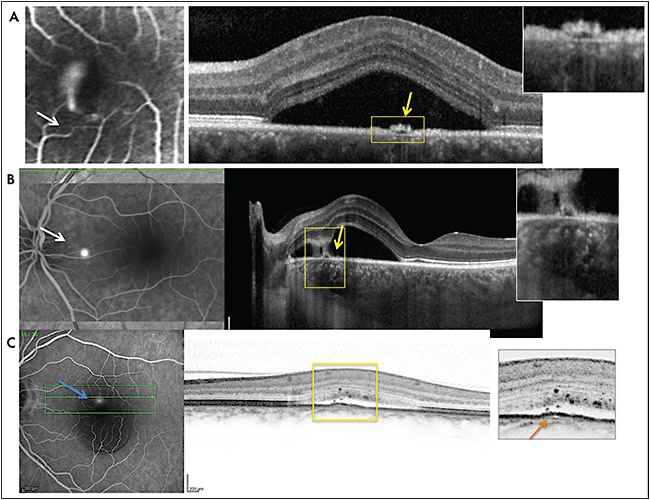
Central serous chorioretinopathy (CSCR) can be considered a retinal phenotypic expression of a larger spectrum of diseases or conditions sharing common physiopathogenic mechanisms. Acute CSCR presents as a serous detachment of the neuroretina due to focal retinal pigment epithelium (RPE) barrier disruption, which allows fliud leakage from an engorged choroid into the subretinal space (Figure 1). Spontaneous healing of the break is usually associated with disease resolution in 3 months to 6 months. Because the RPE is a weak-resistance tight-junction epithelium of only 70-100 ohm.cm2 compared to 1,000 ohm.cm2 for the cornea, it is not surprising that it could break due to various triggering factors (ie, anatomy of choroidal vessels, hormonal changes, choroidal hemodynamic predisposition) and then spontaneously heal. However, a subset of patients has longer first episode duration and/or repeated episodes of the disease that may require therapeutic intervention.
Daruich et al1 showed that episode duration is longer in cases with thicker choroid (subfoveal choroidal thickness [SFCT] ≥500 µm), with higher RPE elevation at leakage sites (≥50 µm), and in older patients (age ≥40 years), suggesting that choroidal vessel pressure on RPE may compromise the normal restoration of RPE barrier. With repeated episodes and imperfect RPE barrier and polarization restoration, diffuse retinal pigment epitheliopathy (also known as chronic CSCR) may occur (Figure 2). Pachychoroid pigment epitheliopathy,2,3 choroidal neovascularization (CNV), or polyps occurring underneath flat irregular pigment epithelium detachments (FIPED)4 can be considered various forms on the same disease spectrum (Figure 2). These different conditions usually share commonalities such as engorged choroid with progressive choroidal vessels remodeling, RPE dysfunction, and alterations without drusen deposits. Although some extraocular factors have been identified as favoring CSCR, they have not been thoroughly analyzed as favoring factors in the other diseases on this spectrum.

Francine Behar-Cohen, MD, PhD is professor of ophthalmology at the Paris Descartes University and Hôtel-Dieu of Paris hospital in Paris, France, professor at University of Lausanne in Switzerland, and director of research at Inserm UMR 1138 at the Centre de Recherche des Cordeliers in Paris, France. Dr. Behar-Cohen reports intellectual property interest in the use of mineralocorticoid receptor antagonists for eye diseases. She can be reached at francine.behar@gmail.com.
The diagnosis of CSCR can be very challenging, being made retrospectively in some older patients after several years of anti-VEGF injections when CSCS is complicated by type 1 CNV.
CSCR OR NOT CSCR?
Some patients are diagnosed with CSCR but have other conditions leading to subretinal fluid (SRF), such as circumscribed choroidal hemangioma, atypical melanocytic tumors, optic disc pit, wet AMD, dome-shaped macula, polypoidal choroidal vasculopathy, or adult-onset vitelliform dystrophy (Figure 3). Some drugs can cause subretinal fluid as well.5 Various and sometimes conflicting results regarding genes known to be associated with AMD, such as complement factor H (CHF) and CSCR, could be due to patients with uncertain diagnostics being included in the cohorts as well as patients with pachychoroid being included in the control group. We have shown that pachychoroid could be the inherited factor predisposing to CSCR, rather than CSCR itself.6 If this is the case, the control cohort should only include patients with subfoveal choroidal thickness <380 µm.

PATIENT HISTORY AND LIFESTYLE CAN GUIDE DIAGNOSIS
CSCR is more common in men (70% to 80%) in their 40s to 50s with a type A7 or ruminant personality, and in women it occurs generally later in life, after menopause. Family history of CSCR, personal previous history of transitory visual disturbance, hypertension, coronary heart disease, seasonal allergy, episode of identified stress, shift work (pilots, transport workers, security agents, police officers, artists, and other creative intellectual occupations),8 sleep disorders particularly if due to circadian disruption,9 and pregnancy are favoring factors. Depression and suicide in personal or family history should be questioned. Glucocorticoids, either endogenous or administered by any routes including local skin or nasal instillations, favor the occurrence and worsen disease severity. They have been found in various series in about 30% of patients, sometimes years before the actual episode.5,10
ROLE OF INFLAMMATION
Because glucocorticoids aggravate rather than improve CSCR, inflammation has been disregarded as a disease mechanism. But in other retinal diseases in which inflammation is recognized as a key player, such as AMD, glucocorticoid treatments also did not show benefit, suggesting that some inflammation may not be corticoid sensitive.11 In CSCR, choroidal engorgement, progressive choroidal alteration with hypereflective foci in the choroid, and in the subretinal space breakdown of the outer retinal barrier and intraretinal fluid occurring in long-lasting disease, as well as choroidal neovascularization could all be considered signs of intraocular inflammation. If CSCR were an inflammatory disease, why should steroids aggravate rather than alleviate the disease?
MINERALOCORTICOID RECEPTOR: A POTENTIAL RECONCILIATORY HYPOTHESIS
The mineralocorticoid receptor (MR) is the ancestral corticoid receptor.12 It was identified in the vessels and the brain in the early 1980s13,14 and in the retina in 1996 by the team of Mirshahi et al.15 But the expression of the receptor itself in a cell or tissue is not sufficient to render the tissue sensitive to mineralocorticoid hormones. Mineralocorticoid receptors have 2 natural ligands, aldosterone and cortisol (or corticosterone, its equivalent in rodents), that bind to it with the same affinity. Glucocorticoid is much more abundant than aldosterone in the circulation. Only in cells that express the enzyme 11-beta-hydroxysteroid dehydrogenase (11ßHSD2), which converts cortisol to cortisone and which has only a very weak affinity for the mineralocorticoid receptor, can aldosterone then bind to its receptor. In most nonepithelial MR targets, 11ßHSD2 is not expressed, and thus glucocorticoids occupy the MR.16
In the kidney, which is the mineral-sensitive organ in essence, upon binding to MR, aldosterone activates the Na+/K+-ATPase to reabsorb sodium and water and excrete potassium. My group, in collaboration with Jaisser et al, has demonstrated that the retina and the choroid are mineral-sensitive tissues, and that overactivation of the receptor by its natural ligand aldosterone induces retinal swelling, and most of the features of acute CSCR in a rat model.17-18 We have identified some ion and water channels as molecular targets of MR in the retina and in the choroid, explaining, at least in part, the mechanism of action of MR pathway activation. Interestingly, in other organs, MR overactivation and/or expression causes disease, inducing endothelial dysfunction, vascular inflammation, metabolic syndrome, vascular fibrosis, and heart fibrotic remodeling after myocardial infarction, which is the actual label for mineralocorticoid receptor antagonists (MRA). In the brain, MR pathway is increasingly recognized as an important player in depression, empathy, and resilience.19 Moreover, Deliyanti et al showed that spironolactone could inhibit retinal neovascularization in the retinopathy of prematurity model.20
From these fundamental works, we have hypothesized that CSCR and other related phenotypes could be the ocular phenotypic expression of MR vascular overactivation (Figure 3) and have proposed oral MRA as a treatment option. Since then, several groups have used MRA for nonresolving CSCR, showing beneficial effects on subretinal fluid (SRF), indicating that MR might control, at least in part, CSCR disease mechanisms and/or have specific effects of retinal hydroionic control.
If the hypothesis of MR pathway overactivation in CSCR patients is valid, it would follow that vascular inflammation and endothelial dysfunction could be aggravated by glucocorticoids and ameliorated by MRA. Therefore, CSCR would be a progressive inflammatory and fibrotic chorioepitheliopathy that would benefit from long-lasting MRA treatment rather than from only acute treatment. Recent findings that peripheral endothelial dysfunction was shown in CSCR patients21 support this hypothesis. Van Dijk et al also recently observed that the CSCR phenotype is frequent in patient with hyperaldosteronism22 and that a polymorphism in the MR gene confers and increased risk of CSCR.23
RECENT ADVANCES IN CSCR DIAGNOSIS
Recent reviews have extensively described the clinical and imaging features of various forms of CSCR.5,24-26 These have focused on OCT angiography (OCTA) because this new imaging modality may change the diagnosis and management of chronic CSCR associated with epitheliopathy. Several recent studies have shown that OCTA is more sensitive than fluorescein angiography (FA) and spectral-domain OCT for diagnosing CNV in FIPED.4,27-28 The recognition of CNV in CSCR with SRF justifies an anti-VEGF treatment challenge. Because CNV can be identified in CSCR without SRF, its exact contribution in SRF accumulation is in question. Findings identified by indocyanin green (ICG) angiography29 will be enhanced by OCTA, which can show alterations of the choriocapillaries and vascular remodeling, providing important information on disease mechanisms.30-32
MANAGEMENT
The CSCR Patient
The management of patients with CSCR should take into account the temperament of the patient as well as environmental factors requiring adjustments, such as shift work, sleep disorders, hypertension and its treatment, as well as other medications. In type A personality patients, visual threats increases anxiety and fear of performance loss. Psychological support could be considered for these patients.

What and When to Treat, and for How Long
Although SRF induces rapid vision loss in other retinal diseases, it does not cause major visual disturbance in CSCR patients, even when it lasts several months. This could result from different retinal changes following detachment. Instead of a shortening of phororeceptor outer segments observed in rhegmatogenous retinal detachment, elongation of segments occurs in CSCR. Visual acuity remains good when the retina reattaches, even after several months, although multifocal ERG functional evaluation remains altered long after retinal reattachment.33,34 But whether longer episode duration is a risk for recurrence has not been specifically addressed, and whether early treatment of acute episodes reduces the risk of diffuse epitheliopathy has not been evaluated. Since argon laser or verteporfin photodynamic treatments carry potential risks of vision loss, it has been recommended to consider them only in cases of sustained subretinal fluid for more than 4 months to 6 months. Whether early treatment with MRA has an impact on the long-term evolution of CSCR remains to be demonstrated.
For chronic CSCR associated with diffuse epitheliopathy, several objectives should guide treatment planning:
- Restore normal retinal anatomy through elimination of macular edema (subretinal or intraretinal fluid) as quickly as possible;
- Prevent recurrence;
- Prevent or reduce permanent RPE alterations, including dedifferentiation (pigment loss, junction loss) and detachments (FIPED and serous PED);
- Prevent and, if possible, restore choroidal vascular alterations including choriocapillaris atrophy, pachyvessel development, and choriocapillaris/RPE interface damage.
To date, no treatment is approved for CSCR. Most studies have concentrated treatment efficacy on 2 endpoints: visual acuity and subretinal fluid decrease. Recent meta-analyses and reviews have extensively analyzed the results of different treatments, with strong evidence that verteporfin photodynamic therapy (PDT) could be beneficial to reduce episode duration and recurrence.35-38
In this article, we focus more specifically on the use of MRA in CSCR. To date, no placebo-controlled randomized study has been conducted to evaluate the long-term effect of MRA in CSCR. Recommendations given here are based on our personal experience with MRA in CSCR. Several studies are currently recruiting patients in randomized trials studying eplerenone for CSCR and results are expected in 1 to 2 years.39-41
The first steroid-competitive MRA drug was spironolactone, approved for the treatment of hypertension in the United States in 1960. It is a potent but nonspecific MRA, as it also interacts with progesterone and with androgen receptors at high doses (>200 mg/day), leading to dose-dependent hormonal side effects such as gynecomastia, erectile dysfunction, a possible decrease in libido, and menstrual irregularities. Eplerenone, designed to reduce the hormonal effects of spironolactone, was approved by the US Food and Drug Administration in 2002. Although eplerenone is a more selective MRA than spironolactone, it is less potent, having a 40-fold lower affinity for the receptor. Both MR antagonists have no effect on testosterone. Regarding their action on electrolytes balance, hyperkalemia is a potential adverse consequence of both spironolactone and eplerenone therapy, but is clinically relevant only in patients with impaired renal function. Plasma potassium concentrations should be monitored at baseline, at 1 week when increasing the dose, and at 1 month. If no change is observed, a 3-month monitoring period is recommended. Caution should be taken not to associate MRA with other potassium-sparing agents or nonsteroidal anti-inflammatory agents. Many drugs can interact with MRA and it is thus recommended to be in contact with the patient’s primary physician to coordinate treatment initiation if the patient is polymedicated. A new nonsteroidal MRA, BAY 94-8862 (Bayer) was designed to reduce hyperkaliemia.41 A treatment scheme was recently published in Translational Vision Science & Technology (Figure 5).42

In patients presenting with FIPED associated with type 1 CNV on OCTA and SRF, 3 anti-VEGF injections at 1-month intervals are recommended. In the absence of SRF decrease with anti-VEGF, MRA could be introduced before proposing PDT and/or in addition to PDT. Regarding the duration of MRA treatment, we have growing preclinical evidence that MR blockade influences major pathways in CSCR pathogenesis, including neoangiogenesis and we are considering it as a long-term treatment rather than only a treatment for acute episodes.
CONCLUSIONS
Central serous chorioretinopathy remains a mysterious disease that has and is still garnering interest. Since 2015, 323 papers have been referenced in PubMed with “central serous chorioretinopathy” as a key word. Many hypotheses are raised and because CSCR is a multifactorial disease, several of them may coexist and contribute each in part to the disease pathogenesis. The use of MRA in CSCR results from translational research. Its place in CSCR treatment remains to be defined by randomized, placebo-controlled trials. The clinical effects of MRA are opening new doors to the understanding of the mineralocorticoid pathway in ocular pathophysiology. Ongoing research should help identify new molecular targets of MRA with potential therapeutic benefit. RP
REFERENCES
- Daruich A, Matet A, Marchionno L, et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. January 6, 2017. [Epub ahead of print]
- Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33(8):1659-1672.
- Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35(1):1-9.
- Hage R, Mrejen S, Krivosic V, Quentel G, Tadayoni R, Gaudric A. Flat irregular retinal pigment epithelium detachments in chronic central serous chorioretinopathy and choroidal neovascularization. Am J Ophthalmol. 2015;159(5):890-903.
- Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118.
- Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. Pachychoroid: an inherited condition? Retina. 2015;35(1):10-16.
- Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7(2):111-131.
- Bousquet E, Dhundass M, Lehmann M, et al. Shift work: a risk factor for central serous chorioretinopathy. Am J Ophthalmol. 2016;165:23-28.
- Setrouk E, Hubault B, Vankemmel F, et al. Circadian disturbance and idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2175-2181.
- Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68(5):329-331.
- Geltzer A, Turalba A, Vedula SS. Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2013;1:CD005022.
- Izumi T, Koizumi H, Maruko I, et al. Mineralocorticoid receptors: evolutionary and pathophysiological considerations. Endocrinology. 2011;152(5):1883-1890.
- Moguilewsky M, Raynaud JP. Evidence for a specific mineralocorticoid receptor in rat pituitary and brain. J Steroid Biochem. 1980;12:309-314.
- Kornel L. Studies on the mechanism of mineralocorticoid-induced hypertension: evidence for the presence of an in-situ mechanism in the arterial wall for a direct action of mineralocorticoids. Clin Biochem. 1981;14(5):282-293.
- Mirshahi M, Nicolas C, Mirshahi A, et al. The mineralocorticoid hormone receptor and action in the eye. Biochem Biophys Res Commun. 1996;219(1):150-156.
- Fuller PJ, Yao Y, Yang J, Young MJ. Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol. 2012;213(1):15-24.
- Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010;24(9):3405-3415.
- Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672-2679.
- Büttner M, Jezova D, Greene B, Konrad C, Kircher T, Murck H. Target-based biomarker selection - mineralocorticoid receptor-related biomarkers and treatment outcome in major depression. J Psychiatr Res. 2015;66-67:24-37.
- Deliyanti D, Miller AG, Tan G, Binger KJ, Samson AL, Wilkinson-Berka JL. Neovascularization is attenuated with aldosterone synthase inhibition in rats with retinopathy. Hypertension. 2012;59(3):607-613.
- Erol MK, Balkarli A, Toslak D, et al. Evaluation of nailfold videocapillaroscopy in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1889-1896.
- van Dijk EH, Nijhoff MF, de Jong EK, Meijer OC, de Vries AP, Boon CJ. Central serous chorioretinopathy in primary hyperaldosteronism. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):2033-2042.
- van Dijk EH, Schellevis RL, van Bergen MG, et al. Association of a haplotype in the NR3C2 gene, encoding the mineralocorticoid receptor, with chronic central serous chorioretinopathy. JAMA Ophthalmol. 2017 Mar 23. [Epub ahead of print]
- Balaratnasingam C, Freund KB, Tan AM, et al. Bullous variant of central serous chorioretinopathy: expansion of phenotypic features using multimethod imaging. Ophthalmology. 2016;123(7):1541-1552.
- Wong KH, Lau KP, Chhablani J, Tao Y, Li Q, Wong IY. Central serous chorioretinopathy: what we have learnt so far. Acta Ophthalmol. 2016;94(4):321-325.
- Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103-126.
- de Carlo TE, Rosenblatt A, Goldstein M, Baumal CR, Loewenstein A, Duker JS. Vascularization of irregular retinal pigment epithelial detachments in chronic central serous chorioretinopathy evaluated with oct angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47(2):128-133.
- Weng S, Mao L, Yu S, Gong Y, Cheng L, Chen X. Detection of choroidal neovascularization in central serous chorioretinopathy using optical coherence tomographic angiography. Ophthalmologica. 2016;236(2):114-121.
- Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151(5);745-751.
- Teussink MM, Breukink MB, van Grinsven MJ, et al. OCT angiography compared to fluorescein and indocyanine green angiography in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2015;56(9):5229-5237.
- Costanzo E, Cohen SY, Miere A, et al. Optical coherence tomography angiography in central serous chorioretinopathy. J Ophthalmol. 2015;2015:134783
- Shinojima A, Kawamura A, Mori R, Fujita K, Yuzawa M. Findings of optical coherence tomographic angiography at the choriocapillaris level in central serous chorioretinopathy. Ophthalmologica. 2016;236(2):108-13.
- Moschos M, Brouzas D, Koutsandrea C, et al. Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica. 2007;221(5):292-298.
- Suzuki K, Hasegawa S, Usui T, et al. Multifocal electroretinogram in patients with central serous chorioretinopathy. Jpn J Ophthalmol. 2002;46(3):308-314.
- Ozkaya A, Alkin Z, Ozveren M, Yazici AT, Taskapili M. The time of resolution and the rate of recurrence in acute central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy: a case-control study. Eye (Lond). 2016;30(7):1005-1010.
- Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841.
- Iacono P, Battaglia Parodi M, Falcomatà B, Bandello F. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res. 2015;55(2):76-83.
- Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014 Dec;92(8):e594-e601.
- NIH Clinical Trials Registry. Eplerenone for central serous chorioretinopathy. Identifier: NCT01822561. Available at: clinicaltrials.gov/ct2/show/NCT01822561 .
- NIH clinical trials registry. A study of the beneficial effects of eplerenone on central serous chorioretinopathy. Identifier: NCT02215330. Available at: https://clinicaltrials.gov/ct2/show/NCT02215330 .
- NHS ISRCTN registry. Clinical efficacy and mechanistic evaluation of Eplerenone for central serous chorio-retinopathy – the VICI randomised trial. Idetnifier: ISRCTN92746680. Available at: www.isrctn.com/ISRCTN92746680 .
- Bärfacker L, Kuhl A, Hillisch A, et al . Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7(8):1385-1403.
- Daruich A, Matet A, Dirani A, et al. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl Vis Sci Technol. 2016 Mar 4;5(2):2.








