Central serous chorioretinopathy (CSC) is characterized by distorted vision (micropsia, metamorphopsia, reduced contrast sensitivity, color desaturation) due to a serous detachment of the neurosensory retina associated with leakage at the (usually impermeable) tight junctions between adjacent retinal pigment epithelial (RPE) cells. The region of exudative retinal detachment in CSC usually occurs in the region of highest choroidal circulation, in the posterior pole of the eye, therefore affecting the central visual field. Spectral-domain optical coherence tomography (SD-OCT) in patients with active CSC typically shows an elevated neurosensory retina above the RPE, which itself is often focally detached over Bruch’s membrane.
PATHOPHYSIOLOGY
Patients with CSC appear to exhibit choroidal vasospasm, mediated by epinephrine, which is potentiated by steroids,1,2 leading to choroidal congestion and pachychoroid (seen on OCT in enhanced-depth imaging mode3-6) and increased choroidal hydrostatic pressure and therefore increased choroidal permeability (demonstrated in CSC patients with indocyanine green angiography7). Increased choroidal vascular permeability leads to increased choroidal interstitial oncotic pressure. Subsequently, serous RPE detachment and hydrostatic overload of RPE tight junctions and resultant RPE leakage lead to exudative neurosensory retinal detachment.
Consistent with the proposed pathophysiology, active CSC has been correlated with elevated levels of endogenous and exogenous corticosteroids. Levels of epinephrine and norepinephrine have also been shown to be elevated in patients with active CSC.8 Experimental intravitreal injections of corticosteroids and experimental intravitreal injections of aldosterone in rats both lead to pachychoroid, hinting that mineralocorticoid receptor activation may be involved in the pathogenesis.9
NATURAL HISTORY
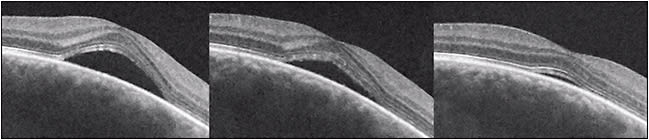
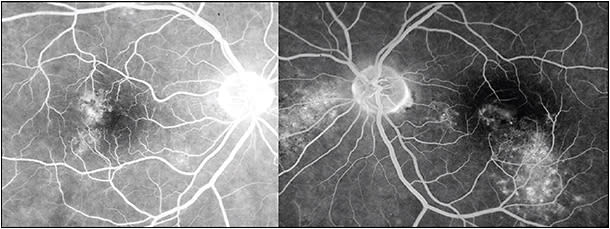
The natural history in the vast majority of CSC cases is spontaneous resolution within 2-3 months (so-called classic CSC). In these cases, central vision is minimally affected and usually returns to normal after reabsorption of the subretinal fluid (Figure 1). However, as many as 10% of all patients with CSC develop persistent serous macular detachment (chronic CSC or “diffuse retinal pigment epitheliopathy” [DRPE], lasting 3 months to 6 months or longer).10 In these DRPE cases, progressive and irreversible visual decline can be associated with the development of central RPE atrophy and (due to increased diffusion distance between the oxygenating and nourishing choriocapillaris, trophic RPE, and the photoreceptors) cystoid macular degeneration and foveal atrophy (Figures 2 and 3).11-15

WHY DO PHOTORECEPTORS FARE BETTER IN EXUDATIVE VS RHEGMATOGENOUS RD?
In experimental retinal detachments, a significant decrease in photoreceptor numbers was observed in a few weeks; no photoreceptors were visible 50 days after retinal detachment.16 However, the external limiting membrane (ELM) and ellipsoid zones (EZs), indicative of photoreceptor integrity, are routinely preserved (or restored) on SD-OCT after spontaneous resolution following a typical, short episode of acute CSC.17
The long survival of photoreceptors in CSC can be explained by the high concentration of glucose and high oxygen tension in the subretinal fluid when the subretinal fluid is from the choroid (as opposed to subretinal fluid derived from the vitreous cavity in rhegmatogenous retinal detachment).18 In fact, it has been shown that oxygen supplementation during experimental retinal detachment reduces the destruction of the photoreceptor elements.19,20
TREATMENT STRATEGY IN CSC
In patients with CSC, it is frequently difficult to discern the exact duration of the episode, so it is prudent to follow the patient’s OCT — specifically the layers indicating photoreceptor integrity: the ELM and the EZ — for disruption or granulation. Treatment prior to photoreceptor disruption would prevent vision loss.
ANTICIPATION OF SPONTANEOUS RESOLUTION
It is likely that those with CSC have a genetic tendency to have choroidal hyperpermeability (perhaps due to increased choroidal flow) induced by steroid exposure, either exogenous or endogenous (Cushing syndrome or stress).21 Initially, the patient should be questioned regarding exogenous steroid exposure (oral, intravenous, intra-articular, inhaled, intranasal or topically applied steroid preparations). In one cohort, 52% of CSC patients had exogenous steroid exposure.22
Discontinuance of the steroid, if possible, will likely result in resolution of the episode. Specific treatment of CSC patients with Cushing syndrome (Figure 3) will also result in resolution of the CSC episode. Also, plasma cortisol levels are elevated during pregnancy, especially during the third trimester. When CSC is associated with pregnancy, resolution of the episode occurs spontaneously after childbirth.
FOLLOW-UP AND TREATMENT OPTIONS
A prospective study that evaluated the effects of CSC on the outer photoreceptor layers by OCT showed that all 3 eyes with episode durations of 3 months or less and foveal reattachment (1 was treated by thermal laser, and the others spontaneously resolved) achieved 20/26 or better (2 of 3 were 20/20) at the final follow-up, and all had preserved outer photoreceptor layers. Conversely, 12 of the other 25 eyes with foveal detachment duration longer than 3 months had a final follow-up visual acuity (VA) of 20/60 or worse, associated with outer photoreceptor layers that were atrophic or not detected.23
Based on these observations, a reasonable approach to the patient with CSC would be monthly follow-up with serial OCT; active treatment should be considered after a CSC episode duration of 3 months. The exact duration of the episode is often impossible to determine, so a conservative approach would include a generous estimate of the duration and prompt initiation of active treatment if disruption of the EZ occurs (disruption of the EZ occurs before ELM disruption).24-26
Also, persistent subretinal fluid in a patient with CSC may be associated with the development of choroidal neovascularization (CNV) or polypoidal choroidal vasculopathy (PCV; which is also associated with pachychoroid) in these patients.27 Also, the appearance of subretinal or sub-RPE hemorrhage in a patient diagnosed with CSC would indicate evolution to CNV or PCV (Figure 4). These developments would necessitate a different treatment approach (prompt intravitreal anti-VEGF treatment or combination intravitreal anti-VEGF and photodynamic therapy [PDT]).28,29
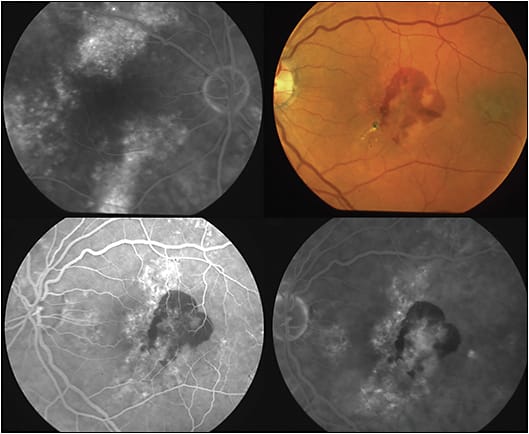
Several active treatment approaches to CSC have been found to be beneficial. Photodynamic therapy with verteporfin (Visudyne) reduces choroidal hyperpermeability, decreases choroidal vascular congestion and thickness, and is effective in the treatment of chronic CSC (Figure 5). Due to concerns of induction of CNV with full (standard) fluence PDT, low-fluence PDT was attempted and was shown to be beneficial.30-35
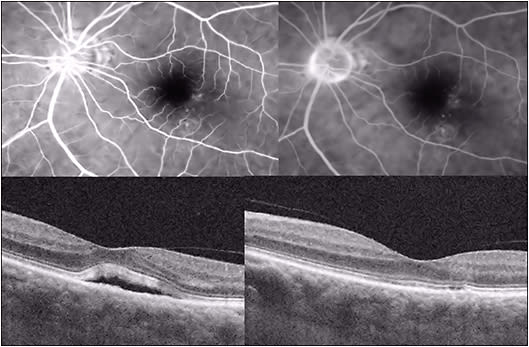
Thermal focal laser photocoagulation of RPE leaks has long been noted to yield improved resorption of subretinal fluid and faster recovery of VA;36 however, because it may be associated with scotoma and induction of CNV, its use recently has been limited to extrafoveal leaks (Figure 6).

Subthreshold diode (810-nm) micropulse laser treatment (“nondamaging retinal laser treatment”) causes expression of heat shock proteins, which may restore RPE function, inhibit apoptosis, and suppress inflammation.37,38
The mineralocorticoid antagonists eplerenone (Inspra; Pfizer) and spironolactone (Aldactone; Pfizer) have shown promise in treating CSC, consistent with the possible role of choroidal mineralocorticoid receptor activation in the pathogenesis of the condition.39,40 These medications should not be used in patients with significant renal disease; careful monitoring of serum potassium levels is indicated during their use, as hyperkalemia may occur.
Rifampin (Rifadin; Sanofi), which hastens steroid metabolism through induction of cytochrome P450 3A4, has been shown to resolve CSC episodes.41-43 Baseline and serial liver function tests are important during rifampin treatment to monitor for potential hepatotoxicity.
Intravitreal anti-VEGF therapy (for antagonism of choroidal hyperpermeability) has been proposed as a treatment for CSC. While intravitreal bevacizumab (Avastin; Genentech) has been unsuccessful,44,45 intravitreal aflibercept (Eylea; Regeneron), which shows higher VEGF affinity, has longer vitreous duration and blocks not only VEGF-A but also VEGF-B and placental-induced growth factor, shows promise.46-50
CONCLUSION
In conclusion, patients found to have CSC should be followed monthly for spontaneous resolution after exposure to corticosteroids (if any exists) has been discontinued. Consideration for active treatment should begin after an episode duration of 3 months (if the beginning of the episode can be ascertained by history), if early disruption of the ellipsoid zone is noted, or if CNV or PCV vasculopathy occurs in association with CSC. Following treatment, recurrence of CSC is noted more frequently in eyes displaying smaller (less than 45 µm) subfoveal choroidal thickness reduction after treatment, indicating more frequent follow-up in these individuals.51 RP
REFERENCES
- Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109(10):1765-1766.
- Tewari HK, Gadia R, Kumar D, et al. Sympathetic–parasympathetic activity and reactivity in central serous chorioretinopathy: a case–control study. Invest Ophthalmol Vis Sci. 2006;47(8):3474-3478.
- Yang L, Jonas JB, Wei W. Choroidal vessel diameter in central serous chorioretinopathy. Acta Ophthalmol. 2013;91(5):e358-e362.
- Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33(2):302-308.
- Manjunath V, Fujimoto JG, Duker JS. Cirrus HD-OCT high definition imaging is another tool available for visualization of the choroid and provides agreement with the finding that the choroidal thickness is increased in central serous chorioretinopathy in comparison to normal eyes. Retina. 2010;30(8):1320-1201; author reply 1321-1322.
- Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469-1473.
- Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203-213.
- Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110(4):698-703.
- Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672-2679.
- Liu DT, Fok AC, Chan W, et al. Central serous chorioretinopathy. In: Ryan SJ, ed. Retina. 5th ed. London: Elsevier Saunders; 2013:1291-1305.
- Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68(11):815-820.
- Yannuzzi LA, Shakin JL, Fisher YL, et al. Peripheral detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91(12):1553-1572.
- Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133(6):787-793.
- Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23(1):1-7.
- Cook B, Lewis GP, Fisher SK, et al. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(6):990-996.
- Erickson PA, Fisher SK, Anderson DH, Stern WA, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24(7):927-942.
- Ojima Y, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Yoshimura N. Restoration of outer segments of foveal photoreceptors after resolution of central serous chorioretinopathy. Jpn J Ophthalmol. 2010;54(1):55-60.
- Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100(3):332-336.
- Sakai T, Lewis GP, Linberg KA, Fisher SK. The ability of hyperoxia to limit the effect of experimental detachment in cone-dominant retina. Invest Ophthalmol Vis Sci. 2001;42(13):3264-3273.
- Mervin K, Valter K, Maslim J, et al. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128(2):155-164.
- Weenink AC, Borsje RA, Oosterhuis JA. Familial chronic central serous chorioretinopathy. Ophthalmologica. 2001;215(3):183-187.
- Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834-1837.
- Piccolino FC, DeLaLongrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139(1):87-99.
- Hagiwara A, Mitamura Y, Kumagai K, et al. Photoreceptor impairment on optical coherence tomographic images in patients with retinitis pigmentosa. Br J Ophthalmol. 2013;97(2):237-238.
- Aizawa S, Mitamura Y, Hagiwara A, et al. Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin Exp Ophthalmol. 2010;38(6):597-604.
- Wakabayashi T, Oshima Y, Fujimoto H, et al. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116(3):519-528.
- Manayath GJ, Shah VS, Saravanan VR, et al. Polypoidal choroidal vasculopathy associated with central serous chorioretinopathy: pachychoroid spectrum of diseases. Retina. 2017 Apr 25. [Epub ahead of print]
- Chan W-M, Lai TYY, Liu DTL, Lam DSC. Intravitreal bevacizumab (Avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol. 2007;143(6):977-983.
- Smretschnig E, Hagen S, Glittenberg C, et al. Intravitreal anti-vascular endothelial growth factor combined with half-fluence photodynamic therapy for choroidal neovascularization in chronic central serous chorioretinopathy. Eye (Lond). 2016;30(6):805-811.
- Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2003; 23(2):235-237.
- Rouvas A, Stavrakas P, Theodossiadis PG, et al. Long-term results of half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Eur J Ophthalmol. 2012;22(3):417-422.
- Bae SH, Heo JW, Kim C, et al. A randomized pilot study of low fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;152(5):784-792.
- Bae SH, Heo J, Kim C, et al. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmology. 2014;121(2):558-565.
- Colucciello M. Choroidal neovascularization complicating photodynamic therapy treatment of central serous retinopathy. Retina. 2006;26(2):239-242.
- Nicholson B, Noble J, Forooghian F, et al. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103-126.
- Robertson DM. Argon laser photocoagulation treatment in central serous chorioretinopathy. Ophthalmology. 1986;93:972-974.
- Wood EH, Karth PA, Sanislo SR, et al. Nondamaging retinal laser therapy for treatment of central serous chorioretinopathy: What is the evidence? Retina. 2017;37(6):1021-1033.
- Ezuddin NS, Lanza NL, Weng CY. Subthreshold micropulse laser photocoagulation in the management of central serous chorioretinopathy. Int Ophthalmol Clin. 2016;56(4):165-174.
- Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33(10):2096-2102.
- Herold T, Prause K, Wolf A, et al. Spironolactone in the treatment of central serous chorioretinopathy–a case series. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1985-1991.
- Steinle NC, Gupta N, Yuan A, et al. Oral rifampin utilisation for the treatment of chronic multifocal central serous retinopathy. Br J Ophthalmol. 2012;96(1):10-13.
- Shulman S, Goldenberg D, Schwartz R, et al. Oral rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(1):15-22.
- Pouw AE, Olmos de Koo LC. Oral rifampin for central serous retinopathy: a strategic approach in three patients. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):98-102.
- Ünlü C, Erdogan G, Aydogan T, et al. Intravitreal bevacizumab for treatment of central serous chorioretinopathy. J Ophthalmic Vis Res. 2016;11(1):61-65.
- Chung Y-R, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond). 2013;27(12):1339-1346.
- Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393-11398.
- Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: a game-changer or quiet addition? Am J Ophthalmol. 2012;154(2):222-226.
- Frampton JE. Aflibercept for intravitreal injection: in neovascular age-related macular degeneration. Drugs Aging. 2012;29(10):839-846.
- Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171-185.
- Yoon YH, Kim DY, Lew Y-J, et al. Intravitreal aflibercept for the treatment of subacute central serous chorioretinopathy: a prospective, randomized study. J Vitreoretinal Dis. 2017;1(2):101-108.
- Kim DY, Joe SG, Yang HS, Lee JY, Kim JG, Yoon YH. Subfoveal choroidal thickness changes in treated idiopathic central serous chorioretinopathy and their association with recurrence. Retina. 2015;35(9):1867-1874.








