The prevalence of nonexudative age-related macular degeneration (AMD) will dramatically increase with an aging population. By the year 2020, an estimated 196 million people globally will have AMD and 11 million will have significant vision loss.1 Whenever cases present with atypical features, ophthalmologists should consider other diagnoses, as numerous retinal diseases have overlapping features with dry AMD. In this article, we present examples of pattern and cone-rod dystrophies, and maculopathies associated with medication toxicity or systemic disorders, all of which were referred to a retina clinic for assessment of dry AMD. These cases were ultimately diagnosed as other pathologies with the aid of ancillary testing. They all can be considered “Dry AMD-masquerade syndromes.”
CASES
Cuticular Drusen (Basal Laminar Drusen)
A diagnosis of cuticular drusen (Figure 1) was originally considered distinct from AMD, with a more favorable prognosis, but more recently it has been considered a subtype of AMD. Despite numerous clinical and histological similarities, important differentiating features exist. On fundus examination, cuticular drusen are small (25-75 microns), yellow-white and nodular. Macular OCT demonstrates these drusen to be blunted, triangular-shaped and below the RPE, giving a saw-tooth pattern.2 FA strikingly reveals innumerable hyperfluorescent drusen that significantly outnumber the drusen noted clinically, yielding the characteristic “stars-in-the-sky” appearance during the early arteriovenous phase.2 Interestingly, on FAF, these drusen are hypoautofluorescent when very small, but also present with a hypoautofluorescent center surrounded by a ring of hyperautofluorescence.2 Overall, the frequency of choroidal neovascularization seen with this AMD subtype ranges from 4% to 56% depending on the study.3-5 One-third of patients can develop geographic atrophy.6 In patients with early-onset, extensive cuticular drusen, a rare complement mutation is associated with an increased risk of developing choroidal neovascularization and type II membranoproliferative glomerulonephritis.4,7,8 Close ocular and systemic monitoring is warranted.
Gass first classified pattern dystrophies, which represent a group of autosomal dominant maculopathies involving pigmentary abnormalities.9 They generally carry a more favorable prognosis than AMD.
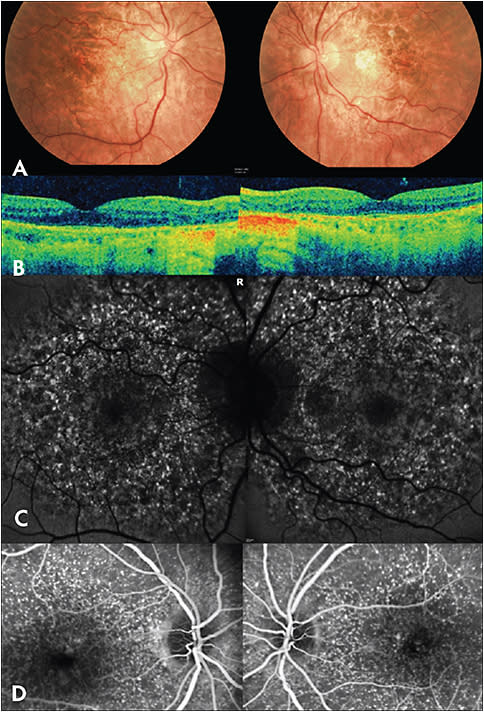
Multifocal Pattern Dystrophy Simulating Fundus Flavimaculatus
Multifocal pattern dystrophy simulating fundus flavimaculatus (Figure 2) is one of five main pattern dystrophy categories that Gass classified.9 Patients develop mild-to-moderate visual disturbances in midlife which progress to severe vision loss in up to 50% of patients after the age 70 due to atrophy of the RPE-photoreceptor complex and/or development of choroidal neovascularization.10 Early in the course of the disease, patients have nonspecific pigmentary changes.4 Subsequently, they develop a variable number of irregularly shaped and/or elgongated, yellow-white flecks throughout the posterior pole and around retinal vascular arcades.10 FA reveals staining of these lesions and FAF demonstrates significantly increased autofluorescence of the flecks with a small, adjacent zone of decreased autofluorescence.10 Macular OCT shows moderately reflective deposits just anterior to the ellipsoid zone.11 Most patients have an abnormal electrooculogram and 50% have an abnormal ERG.10 Genetic testing can reveal a peripherin/RDS mutation but other mutations have been associated with this pathology.10

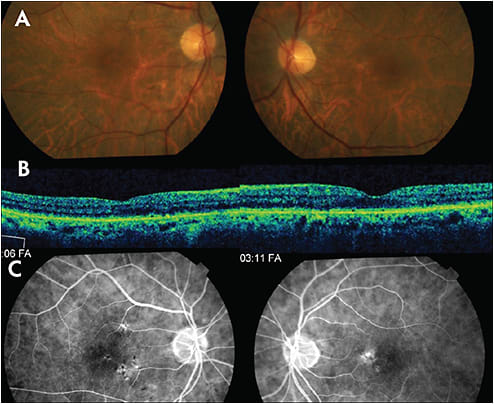
Adult-Onset Foveomacular Vitelliform Dystrophy
Adult-onset foveomacular vitelliform dystrophy (Figure 3), another pattern dystrophy, typically presents with mild visual disturbances between 30 and 50 years of age.12 Vitelliform lesions are hyperautofluorescent on FAF and hypofluorescent on early FA frames with later staining.13,14 Macular OCT reveals a dome-shaped hyperreflective area between the RPE and ellipsoid zone.12,15 Genetic testing classically identifies a mutation in the peripherin 2 (PRPH2) gene, but other genetic mutations have been associated.13

Cone-rod dystrophy
Cone-rod dystrophy (Figure 4) typically presents during childhood with poor VA and severe color-vision loss.16 Fundus appearance can range from normal to a bull’s eye maculopathy with temporal pallor of the optic nerve.16 Goldman visual fields reveal a central scotoma.16 Full-field ERG, which is necessary for diagnosis, demonstrates progressively deteriorating cone amplitudes with comparatively normal rod amplitudes.16

Hydroxychloroquine Toxicity
Hydroxychloroquine is used for many rheumatologic disorders. The toxicity risk is very low in patients who consume less than 5.0 mg/kg of real body weight and who have been on therapy for less than 10 years.17 When toxicity develops (Figure 5), patients frequently complain of nyctalopia and paracentral scotomas. This paracentral scotoma can be identified and tracked with visual fields, the most sensitive of which is a 10-2 Humphrey visual field. Early in the course of the disease, fundus appearance is normal and in later stages a bull’s eye maculopathy develops. Macular OCT reveals a loss of the external limiting membrane, disruption of the ellipsoid zone, parafoveal thinning of the outer nuclear layer, and RPE damage.18 On multifocal ERG, paracentral, central or generalized amplitude reductions develop.19 FAF findings vary depending on disease severity: early pathology highlights a paracentral ring of increased autofluorescence; moderate severity shows a paracentral mottled hypoautofluorescence with an adjacent hyperautofluorescence; and advanced disease reveals a complete central loss of autofluorescence.19

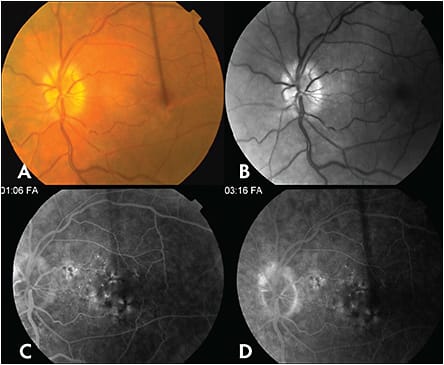
Cancer-Associated Retinopathy
Cancer associated retinopathy (Figure 6) typically presents with subacute vision loss over weeks to months.20 Symptoms vary depending on the degree of rod and cone involvement; patients frequently present with symptoms of shimmering or flickering lights.20 On clinical examination, patients show a normal appearing fundus early in the course of the disease, but with progression, they develop retinal arteriolar attenuation, retinal pigment epithelial mottling and optic disc pallor.20,21 Goldman visual field testing can identify a generalized depression or a central, paracentral, arcuate or ring scotoma.22 ERG is a sensitive diagnostic test early in the course of disease and demonstrates a depression of a- and b-waves on either phototopic and/or scotopic conditions depending on the degree of rod or cone involvement.22 FAF can reveal abnormal hyperautofluorescence surrounding a parafoveal region of normal autofluorescence.23 Macular OCT shows a loss of outer retinal complex components.23 Immunohistochemistry can identify antiretinal antibodies, most common of which are antirecoverin and anti-alpha-enolase antibodies.20
Myotonic Dystrophy
The classic ocular finding in myotonic dystrophy is the “Christmas tree cataract,” a posterior subcapsular, red and green, iridescent opacification of the lens. Patients can present with other ophthalmic findings including ocular hypotony and pigmentary retinopathy (Figure 7) that can be confused for AMD.24,25 The pathognomonic systemic feature is muscle weakness and myotonia of the distal legs, hands, neck and face.26 Fundus examination can reveal butterfly-shaped macular pigmentary changes, peripheral reticular pigmentation and peripheral polygonal-shaped atrophy.27 FAF of the macular pigmentary changes demonstrates a branching linear pattern of mixed hyper- and hypoautofluorescence.28 Macular OCT of these changes identifies patches of inderdigitation zone hyperreflectivity and ellipsoid zone hyporeflectivity.28
CONCLUSION
Despite the prevalence of dry AMD, it is important for ophthalmologists to consider less common diseases and multi-modal imaging to arrive at the correct diagnosis. As demonstrated in this series, numerous pathologies have overlapping features with dry AMD, including pattern dystrophies, cone-rod dystrophies, medication toxicities, and systemic disorders associated with maculopathies. Other common disorders with overlapping features of dry AMD include inactive central serous retinopathy and other “pachychoroid”-related maculopathies, particularly in older patients, as these entities can cause macular pigmentary changes. Historical and exam features that raise suspicion of “Dry AMD-masquerade syndromes” include younger age, suspect medications and systemic disorders, family history, highly symmetric macular pathology and lack of typical drusen. Cuticular drusen and flecks, as demonstrated above, as well as dominant drusen (Malattia Leventinese), and even crystalline retinopathies such as tamoxifen maculopathy, could mimic drusen. Ancillary testing with red-free photography, FA, FAF, macular OCT and ERG can be helpful in further characterizing the retinal pathology. Newer techniques such as OCT with enhanced depth imaging can be used to demonstrate thickened choroid, which is characteristic of central serous retinopathy and other “pachychoroid”-related maculopathies. In the future, genetic testing will become commonplace, and will further delineate many of these maculopathies. RP
REFERENCES
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global health. 2014;2(2):e106-116.
- Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina (Philadelphia, Pa). 2010;30(9):1441-1454.
- Boon CJ, van de Ven JP, Hoyng CB, den Hollander AI, Klevering BJ. Cuticular drusen: stars in the sky. Progress in retinal and eye research. 2013;37:90-113.
- Boon CJ, Klevering BJ, Hoyng CB, et al. Basal laminar drusen caused by compound heterozygous variants in the CFH gene. American journal of human genetics. 2008;82(2):516-523.
- Cohen SY, Meunier I, Soubrane G, Glacet-Bernard A, Coscas GJ. Visual function and course of basal laminar drusen combined with vitelliform macular detachment. The British journal of ophthalmology. 1994;78(6):437-440.
- Khan KN, Mahroo OA, Khan RS, et al. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Progress in retinal and eye research. 2016;53:70-106.
- Leys A, Vanrenterghem Y, Van Damme B, Snyers B, Pirson Y, Leys M. Fundus changes in membranoproliferative glomerulonephritis type II. A fluorescein angiographic study of 23 patients. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1991;229(5):406-410.
- van de Ven JP, Boon CJ, Fauser S, et al. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Archives of ophthalmology (Chicago, Ill : 1960). 2012;130(8):1038-1047.
- Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. Mosby; 1997.
- Boon CJ, van Schooneveld MJ, den Hollander AI, et al. Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. The British journal of ophthalmology. 2007;91(11):1504-1511.
- Roy R, Kumar S, Chandrasekharan DP, Ghose A, Sharma P. Multimodal imaging in multifocal pattern dystrophy simulating fundus flavimaculatus. Indian journal of ophthalmology. 2016;64(5):395-396.
- Rocha Bastos R, Ferreira CS, Brandao E, Falcao-Reis F, Carneiro AM. Multimodal Image Analysis in Acquired Vitelliform Lesions and Adult-Onset Foveomacular Vitelliform Dystrophy. Journal of ophthalmology. 2016;2016:6037537.
- Chowers I, Tiosano L, Audo I, Grunin M, Boon CJ. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Progress in retinal and eye research. 2015;47:64-85.
- Grob S, Yonekawa Y, Eliott D. Multimodal imaging of adult-onset foveomacular vitelliform dystrophy. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2014;28(2):104-110.
- Querques G, Forte R, Querques L, Massamba N, Souied EH. Natural course of adult-onset foveomacular vitelliform dystrophy: a spectral-domain optical coherence tomography analysis. American journal of ophthalmology. 2011;152(2):304-313.
- Thiadens AA, Phan TM, Zekveld-Vroon RC, et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology. 2012;119(4):819-826.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA ophthalmology. 2014;132(12):1453-1460.
- Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clinical ophthalmology (Auckland, NZ). 2010;4:1151-1158.
- Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Investigative ophthalmology & visual science. 2006;47(8):3531-3538.
- Khan N, Huang JJ, Foster CS. Cancer associated retinopathy (CAR): An autoimmune-mediated paraneoplastic syndrome. Seminars in ophthalmology. 2006;21(3):135-141.
- Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. American journal of ophthalmology. 2014;157(2):266-272.e261.
- Rahimy E, Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Survey of ophthalmology. 2013;58(5):430-458.
- Pepple KL, Cusick M, Jaffe GJ, Mruthyunjaya P. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. The British journal of ophthalmology. 2013;97(2):139-144.
- Ikeda KS, Iwabe-Marchese C, Franca MC, Jr., Nucci A, Carvalho KM. Myotonic dystrophy type 1: frequency of ophthalmologic findings. Arquivos de neuro-psiquiatria. 2016;74(3):183-188.
- Rosa N, Lanza M, Borrelli M, et al. Low intraocular pressure resulting from ciliary body detachment in patients with myotonic dystrophy. Ophthalmology. 2011;118(2):260-264.
- TD. B. Myotonic Dystrophy Type 1. In: Pagon RA, Adam MP, Ardinger HH, et al, editors GeneReviews® [Internet]. 1999 Sep 17 [Updated 2015 Oct 22].(Seattle (WA): University of Washington, Seattle; 1993-2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1165/ ).
- Kimizuka Y, Kiyosawa M, Tamai M, Takase S. Retinal changes in myotonic dystrophy. Clinical and follow-up evaluation. Retina (Philadelphia, Pa). 1993;13(2):129-135.
- Esteves F, Dolz-Marco R, Hernandez-Martinez P, Diaz-Llopis M, Gallego-Pinazo R. Pattern dystrophy of the macula in a case of steinert disease. Case reports in ophthalmology. 2013;4(3):129-133.








