Visualization of the peripheral retina has become essential to the screening, diagnosis, monitoring, and treatment of many vision-threatening eye diseases. Early diagnosis of retinal or choroidal disease through diagnostic imaging could reduce potential vision loss. This review discusses the various imaging modalities to perform a widefield angiography examination and their application in the clinical setting of several diseases.
In 1926, the Carl Zeiss company produced the first fundus camera with 20° field of view of the retina.1 The standard field of view was defined as 30°, which is useful to examine the posterior pole. Subsequent improvements in technology allowed better visualization of the peripheral retina, overcoming previous difficulties like limited depth range, optical distortions, and low image quality. Imaging angles larger than the standard reference have been referred to as ‘‘widefield’’ and could be used to analyze retinal periphery, and the term “ultrawidefield” has been reserved for the description of capturing 200° of field of view in a single image.2
Indocyanine green angiography and fluorescein angiography use a water-soluble dye to visualize choroidal and retinal vasculature, respectively.3 The longer wavelengths of the indocyanine green angiography dye allow for better penetrance of ocular structures (more so than fluorescein angiography), making it the best modality to image the choroidal vasculature.4 The advent of widefield fluorescein angiography and indocyanine green angiography enabled visualization of the vasculature in both the central and peripheral retina in a single examination.
In clinical practice, several imaging systems can be used for angiographic examination to screen, diagnose, monitor, and treat many vision-threatening eye diseases. Each has its advantages and disadvantages related to the need for a contact lens, pupillary dilation, location and mechanism of the light source, and the use of traditional photography as opposed to confocal scanning laser.5
CLASSIC FUNDUS CAMERA
Classic fundus cameras, low-power microscopes with a camera attached, provide a magnified view of the fundus.6 The field of view ranges from 30° to 60°. The optical system is based on an aspheric objective lens aligned with a 35-mm single-lens reflex lens system to allow the photographer an exact view of the image to be captured.5 To obtain a wider view of the fundus, several photographs could be taken using a lens with a small field of view and combined to create a montage to investigate the peripheral retina.
Performing fluorescein angiography with this device could be challenging because images of different areas are not taken at the same time and comparison is not suitable, the far periphery of the retina/choroid is not properly visualized, and excellent pupillary dilation as well as patient cooperation with moving the eye in several directions are needed. Also, special filters are required to image the dye during angiography.
EQUATOR-PLUS
In 1975, Pomerantzeff designed the Equator-plus camera, a widefield device for imaging up to 148° of the retina from equator to equator and slightly beyond.7 This system required an appropriate pupil dilation, a contact lens, and fiberoptic transpupillary illumination or scleral transillumination as this device separated the illumination source from the camera observation aperture.7 Images were good but had limited resolution due to the brilliance of light at the site of the transillumination.
RETCAM
The RetCam system (Natus Medical, Inc.) utilizes a contact lens and fiberoptic illumination allowing the performance of widefield images of the retina.8 Several contact lenses can be used to visualize periphery with different degrees up to 130°. Images are obtained using illumination performed through the central cornea, the anatomic lens, and the dilated pupil.8 Lens opacity and inadequate pupil dilation produced poor image quality. The device is portable and easily transported to the bedside, allowing the examination of the retina for patients unable to place themselves in front of a fundus camera. It has been used to image pediatric patients, offering good visualization of retinal pathological features.9-12
PANORET
The Panoret-1000 (Medibell Medical Vision Technologies Ltd.) obtains a 130° panoramic view of the retina with image resolution of 20 micrometers and little glare or scleral brightness at the illumination site.13 It utilizes a contact lens and fiberoptic or scleral transillumination, allowing the investigation of retinal periphery.13 Fluorescein angiography and indocyanine green angiography can be performed using 1024 x 1024-pixel charge-coupled–device camera, acquiring at a video rate for focus and alignment with 3 high-quality images saved per second, and without pupil dilation.13 For imaging, one operator uses one hand to hold the camera, one hand to hold the light, and a foot to capture the image, and a second operator uses the computer.13 Focusing and recording images required adjustments made on the computer, and image quality prior to recording was not predictive of the saved image. This imaging technique can be performed in patients with small pupils, cataract, or an intraocular lens implant because the light is delivered posterior to these abnormalities. Image quality depends on placement and direction of the external light source, the type of ocular pathology, and to some extent the degree of eye pigmentation, because the trans-scleral illumination was dimmed in patients with heavily pigmented uveas.13
OPTOS
The Optos (Optos plc) imaging system is a scanning laser ophthalmoscope that can image up to 200° (120° on horizontal axis and 80° on vertical axis) of the ocular fundus in a single image without the need for a contact lens or pupillary dilation.14 This tool utilizes the optics of an ellipsoid mirror that contains two focal points. The laser of the Optos is directed through one of the focal points, while the patient’s eye is positioned so that the second focal point is located inside the patient’s eye.15 Their latest device, the California ultrawidefield retinal imaging device, offers a comprehensive retinal analysis through multiple wavelengths, the green (532 nm), red (635 nm), infrared (802 nm), and blue (488 nm), allowing concomitant fluorescein angiography and indocyanine green angiography examination without manually switching between imaging modalities. Panoramic fluorescein and indocyanine green angiography are acquired rapidly by single capture using this device, in contrast to other panoramic images composed manually or with computer software, which have difficulties with imaging the peripheral retina, illumination irregularities, and time gaps among individual pictures. There are issues with reflections from eye astigmatism, the cornea, and eye movements. The device has low posterior pole resolution compared to standard fundus cameras and high-resolution confocal scanning laser ophthalmoscopes, such as the Heidelberg Spectralis.
CONFOCAL SCANNING LASER OPHTHALMOSCOPE
Simultaneous widefield fluorescein and indocyanine green angiography with a confocal scanning laser ophthalmoscope can be performed to detect and record areas of peripheral pathology, such as chorioretinal nonperfusion and neovascularization, beyond the range of conventional fundus cameras. Several compatible lenses in the Spectralis confocal scanning laser ophthalmoscope (Heidelberg Engineering) can be used to obtain different fields of view.
Staurenghi et al reported the use of an integrated, multielement contact lens-based system (Ocular Staurenghi 230 SLO Retina Lens; Ocular Instruments, Inc.).16 The integrated system consists of 2 biconvex aspheric lenses and a 2-element convex-concave contact lens. It has 0.23 magnification and is afocal when used with gonioscopic gel increasing the field of view fivefold and up to 150°. Antireflection coatings reduce reflections to less than 0.1% for 514-nm red-free and 835-nm infrared reflectance images.16 Simultaneous widefield fluorescein and indocyanine green angiography could be performed with the 2 laser wavelengths of this device: 488 nm for fluorescein angiography and 795 nm for indocyanine green angiography. Disadvantages of this imaging technology include the need for topical anesthesia prior to placing the contact lens, patients’ cooperation during the examination, and the need for a skilled photographer to hold the lens while obtaining images. The images obtained are inverted and reversed, but the camera’s software can restore the correct orientation.
Alternately, the Ocular Lee-Mainster SLO Lens (Ocular Instruments, Inc.) is designed for the HRA 2 to investigate peripheral retina with 60° of view. Optics are specially coated to reduce reflections and provide enhanced image contrast during fluorescein and indocyanine green angiography. Images are obtained easily in a noncontact manner.
More recently, Heidelberg Engineering developed a noncontact ultrawidefield angiography module for the Spectralis and HRA 2. On these devices, the 30° and 55° noncontact lens can be attached to the camera head to produce fluorescein and indocyanine green angiographic images. Moreover, the new module consists of an interchangeable noncontact lens that attaches to the camera head to provide high-contrast, undistorted, and evenly illuminated images out to the peripheral retina with 102° of view. Both fluorescein and indocyanine green angiography, individually or simultaneously, may be performed with this module (Figure 1). Several reports have demonstrated the utility of widefield angiography imaging in screening, diagnosis, monitoring, and treatment of many peripheral diseases.17
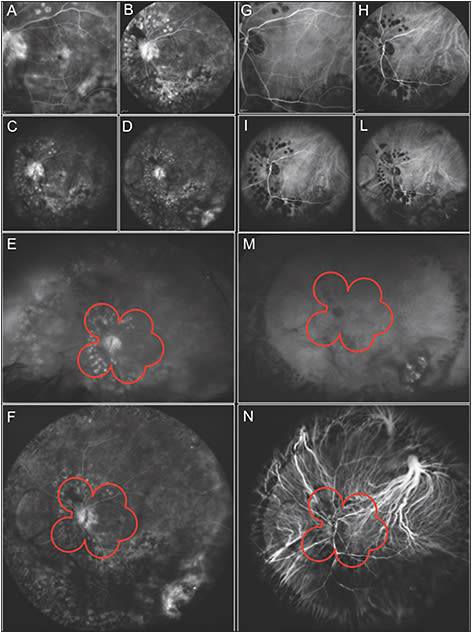
NORMAL SUBJECTS AND PATHOLOGIC MYOPIA
Evaluation of peripheral angiographic findings in pathologic conditions should be compared to subjects with normal peripheral retinal anatomy as a reference standard. A recent report evaluated the angiographic findings in patients without peripheral disease showing high prevalence of peripheral vascular anatomic variations.18 The prevalence of missing capillary details and presence of ground glass hyperfluorescence were high. However, it remains unclear whether the missing capillary detail was due to capillary nonformation or a secondary loss of capillaries.18
Moreover, Kaneko et al19 evaluated 115 eyes with pathologic myopia compared to 42 emmetropic eyes showing large areas of nonperfusion by capillaries and large vessels, telengectasia, and microaneurysms in myopic eyes by using widefield fluorescein angiography.19 These last two findings were present in both emmetropic and highly myopic eyes, even if the severity score of the lesions was different.19 Widefield fluorescein angiography has proven to be very useful in distinguishing healthy capillaries from those with pathology. The coexistence of dye leakage from microaneurysms and dilated capillaries in the areas of ramified patterned capillaries could suggest the presence of capillary telangiectasia. Furthermore, widefield fluorescein angiography enabled identification of avascular peripheral area in 82.6% of eyes with pathologic myopia, and in only 4.8% of emmetropic eyes, suggesting that peripheral avascularity was a characteristic of eyes with pathologic myopia.19 Pathologic myopia is characterized by an excessive elongation of the axial length and by an altered shape of the entire eye.20,21 These findings indicate that the lesions associated with pathologic myopia were not confined to the posterior pole of the eye and were present the peripheral areas of the retina.
AGE-RELATED MACULAR DEGENERATION
Age-related macular degeneration is the leading cause of irreversible blindness among the elderly.22 Mechanisms such as hypoxia and ischemia have been proposed to explain the pathogenesis of AMD, which is characterized by the loss of photoreceptors, retinal pigment epithelium, and choriocapillaris in the central macula.23 Madhusudhan et al investigated the perfusion of the peripheral retina in neovascular AMD using widefield fluorescein angiography to determine if peripheral retinal ischemia might contribute to development of the disease.24 A total of 30 eyes with neovascular AMD were enrolled, finding areas of peripheral retinal nonperfusion in 2 eyes (7%), peripheral vascular leakage in 5 eyes (17%), and diffuse dye leakage close to the ora in 5 eyes (17%).24 Peripheral retinal nonperfusion did not appear to be associated with neovascular AMD while peripheral leakage and diffuse hyperfluorescence were present, and these elements well correlated to degeneration of blood retinal barrier and loss of RPE integrity.24 Moreover, peripheral leakage, as seen on widefield fluorescein angiography, was associated with active neovascular AMD in a proportion of patients compared to the fellow eyes without active neovascular AMD.24
DIABETIC RETINOPATHY
Diabetic retinopathy is characterized by increased vascular permeability, retinal ischemia, edema, and formation of new vessels.25 It is one of the leading causes of blindness among adults.26 Ischemic changes and microvascular abnormalities in patients with DR have long been hypothesized to play a role in the development of DME despite that DR is frequently located in the midperiphery.27,28 The Diabetic Retinopathy Study introduced imaging of DR in the retinal periphery by obtaining seven standard fields.29 By combining these 30° images, a montage visualizes about 75° of the retina.29 In particular, fluorescein angiography is a useful imaging technique to identify areas of capillary drop-out, nonperfusion, and leakage from retinal neovascularization. Several studies have demonstrated the association between peripheral retinal nonperfusion and the occurrence of neovascularization and DME.30-32 Interestingly, Wessel et al31 compared the areas of retinal pathology visualized by Optos ultrawidefield fluorescein angiography with those visualized by conventional ETDRS standard 7-field imaging. Ultrawidefield fluorescein angiography images captured 3.9 times more area of retinal nonperfusion, 1.9 times more neovascularization and 3.2 times more retinal surface area than that seen within the ETDRS standard 7-field overlay.31 Kimble et al32 found that capillary nonperfusion was detected in 84% of patients with DME. In their study population, late leakage from peripheral retinal vessels in areas of nonperfusion was associated with ME and related to formation of new vessels.32
Oliver et al33 evaluated the angiographic features of 264 eyes of 143 patients with DR, finding a direct correlation between capillary nonperfusion and late leakage from peripheral retinal vessels by ultrawidefield fluorescein angiography. Peripheral vessel leakage was detected as frequently as neovascularization (41% of eyes) and less frequently than peripheral nonperfusion (54% of eyes) or ME (57% of eyes).
Another important application of widefield fluorescein angiography in DR is the evaluation of peripheral retinal areas of nonperfusion or neovascularization to perform a target laser photocoagulation. This technique is useful to reduce vascular endothelial growth factor production, increase oxygen diffusion from the choroid, reduce the formation of ME, and therefore save visual acuity.34,35 Widefield fluorescein angiography allows to treat specific areas of retinal nonperfusion using less energy and sparing relatively better perfused tissue from laser-induced tissue scarring (Figure 2).

RETINAL VEIN OCCLUSION
Retinal vein occlusion is the most prevalent blinding vascular retinal disorder after DR.36 Retinal venous occlusions may lead to peripheral retinal ischemia and neovascularization. Ultrawidefield fluorescein angiography is a useful imaging technique to evaluate peripheral retinal areas of nonperfusion and neovascularization.37 Prasad et al found a direct correlation between peripheral nonperfusion and ME with neovascularization. Ultrawidefield fluorescein angiography may be a powerful tool to identify therapeutic target areas for photocoagulation, allowing for efficient treatment of ischemic retina, and potentially minimizing collateral destruction of adjacent viable perfused retina (Figures 3 and 4).
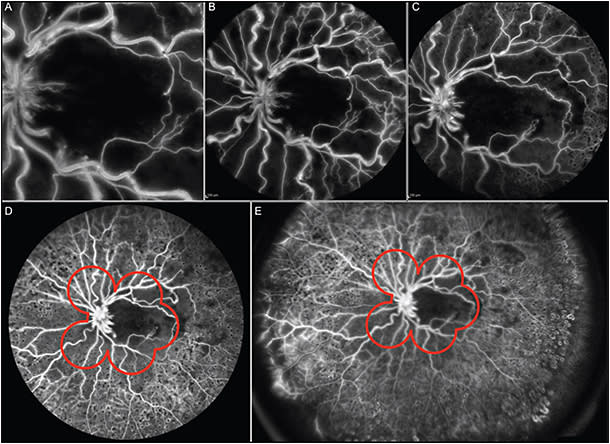

Recently, Spaide evaluated peripheral nonperfusion with widefield angiography in eyes with central retinal vein occlusion treated with anti-VEGF agents and found an inverse correlation between the area of peripheral nonperfusion and visual acuity.38 Widefield angiography, therefore, is capable of documenting peripheral nonperfusion before and after anti-VEGF therapy.38
UVEITIS
Accurate diagnosis, definitions of activity, quiescence, and response to treatment for uveitis are typically defined by clinical and angiographic appearance.39 Angiographic examinations are used to determine diagnosis, severity, progression, and response to therapy as well as detect complications such as ischemia and neovascularization.39 Some features of posterior uveitis, such as perivascular sheathing, exudates, hemorrhages, peripheral capillary nonperfusion, venous staining or leakage, cystoid ME, and disc edema, could be detected by widefield fluorescein or indocyanine green angiography.40 This detects images of both central and peripheral retina simultaneously giving more precise details in the montage reconstruction of images.40 Reeves et al reported a series of 26 patients with retinal vasculitis that underwent a fluorescein angiography with the Staurenghi lens system, finding vasculitis not clinically evident in 31% of patients and significant nonperfusion areas in a third of patients, allowing diagnosis and characterization of the extent of the disease in 62%.40 They also found widefield angiography particularly useful in guiding peripheral laser treatment.
Tsui et al performed an ultrawidefield angiographic study finding eyes with central large-caliber venous staining and leakage, while others had peripheral small-venule staining and leakage. These two findings were called central and peripheral phlebitis patterns.41 With ultrawidefield angiography, the authors also noticed that venous abnormalities affected both the superior and inferior fundus with the same frequency, even though snowbanking occurs inferiorly most often.41 The authors could evaluate the response to treatment and detect and localize inflammation, describing whether it follows a linear pattern along the vascular tree, or if it has a multifocal pattern.41
Karampelas et al investigated in 82 consecutive patients with uveitis the relationships between peripheral vasculitis, ischemia, and vascular leakage using ultrawidefield fluorescein angiography.42 Peripheral focal vasculitis and diffuse capillary leakage were shown to be important markers of disease activity, as they were associated with the development of ME and neovascularization.41 In particular, peripheral ischemia was correlated with neovascularization-related leakage while none of the peripheral changes to the vascular bed had any associations with visual acuity.42
Interestingly, a correlation was observed between peripheral ischemia and focal peripheral vasculitis, but not with diffuse capillary leakage, suggesting that focal large-vessel vasculitis is more closely related to the development of ischemia, which has potential implications for the clinical management.42 Nonperfused areas may not always represent disease activity, whereas it is a well-established fact that vessels next to areas of ischemia frequently exhibit angiographic leakage.42 The use of ultrawidefield fluorescein angiography with the Optos device facilitates examination because the intensity of light is more tolerable for the patient, fewer images are necessary to identify the retina, and the optics enable imaging even through small pupils despite corneal or vitreous artifacts, which can modify image resolution.42,43
PEDIATRIC RETINAL DISEASE
In pediatric patients, binocular indirect ophthalmoscopic examination requires a skilled examiner and is not without risk because of the potential to induce cardiorespiratory complications.44,45 The importance of fundus fluorescein angiography of the peripheral retina in infants with retinal vasculopathies such as ROP, FEVR, and incontinentia pigmenti is well recognized for the diagnosis of neovascularization, capillary nonperfusion, and leakage.46
RetCam was a widely available imaging modality used to perform fluorescein angiography on a supine infant under general anesthesia. Peripheral sweeps and montage techniques were often necessary and did not permit the simultaneous capture of the posterior pole and periphery in a single frame, limiting the evaluation and comparison of the phases of the angiogram across different parts of the fundus.45 Moreover, this device has the potential to cause retinal hemorrhages, induce an oculocardiac response, and possibly affect retinal artery perfusion from ocular compression.47-49
The panoramic P200MA system (Optos PLC) is a valid alternative to the RetCam to perform a noncontact ultrawidefield oral and intravenous fluorescein angiography with no sedation during the procedure.49,50 This device was reliable and efficient in evaluating the peripheral retina of small-sized infants with various retinal vasoproliferative disorders.50,51 However, the angiographic images obtained from the P200MA were often inadequate in larger and older infants.46 In these case and when angiography is required under general anesthesia in uncooperative infants, ultrawidefield angiography with the Spectralis has several potential advantages.46 There is no contact with the ocular surface, no subsequent compression artifact, and no photomontages avoiding skipped areas and local variations in magnification and contrast. Spectralis produces images of superior quality at lower retinal irradiances, allowing a greater sensitivity for detecting vascular leakage and areas of capillary nonperfusion.46,52,53
In a recent report, the authors demonstrated the utility of ultrawidefield oral fluorescein angiography for studying infants with incontinentia pigmenti.54 It was possible to identify the retinal features of this disease, including areas of retinal ischemia, peripheral retinal neovascularization, and arteriovenous shunts.54
Moreover, ultrawidefield fluorescein angiography can be used to evaluate Coats disease, an idiopathic retinal vascular abnormality of young males characterized by telangiectatic retinal vessels with aneurysms.55-57 Abnormal permeability of these vessels leads to exudative retinal detachment and subretinal lipid deposits. This technique permits the visualization of a widefield image of the retina and an accurate identification of the pathologic areas assisting planning laser treatment, reducing unnecessary photocoagulation, and minimizing laser-related scarring and potential visual loss in the long term.57
CONCLUSIONS
Imaging of the peripheral retina has significantly improved. Several devices may be used, each with its advantages and disadvantages. Widefield angiographic technology has become important clinically with regards to early diagnosis, treatment, and monitoring of most sight-threatening retinal diseases.
Widefield angiography imaging offers a larger retinal view compared to the ETDRS standard 7-field photos, obtaining more details of retinal nonperfusion, neovascularization, and surface area. The advent of widefield fluorescein angiography and indocyanine green angiography have enabled visualization of both the central and peripheral retina vasculature in a single examination. This is very useful in the evaluation of peripheral retinal areas of nonperfusion or neovascularization to perform a target laser photocoagulation. Widefield fluorescein angiography obtains an enhanced visualization of different areas taken at the same time, allowing less energy and sparing relatively better-perfused tissue from laser-induced tissue scarring.
Widefield angiography will continue to increase in clinical and research settings as there are several ocular diseases with peripheral retinal findings that need standardized description and characterization. RP
REFERENCES
- Ciardella A, Brown D. Wide-field imaging. In: Agarwal A, editor. Fundus Fluorescein and Indocyanine Green Angiography: A Textbook and Atlas. Thorofare, NJ: Slack Inc; 2007:79-84.
- Soliman AZ, Silva PS, Aiello LP, Sun JK. Ultra-wide field retinal imaging in detection, classification, and management of diabetic retinopathy. Semin Ophthalmol. 2012;27(5-6):221-227.
- Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Invest Ophthalmol. 1973;12(4):248-261.
- Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6(3):25-32.
- Witmer MT, Kiss S. Wide-field imaging of the retina. Surv Ophthalmol. 2013;58(2):143-154.
- Saine P, Tyler M. Ophthalmic Photography: A Textbook of Retinal Photography, Angiography and Electronic Imaging. Boston, MA: Twin Chimney Publishing; 1997.
- Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975;14(5):401-406.
- Dhaliwal C, Wright E, Graham C, McIntosh N, Fleck BW. Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. Br J Ophthalmol. 2009;93(3):355-359.
- Koozekanani DD, Connor TB Jr, Wirostko WJ. Retcam II Fluorescein angiography to guide treatment and diagnosis of Coats disease. Ophthalmic Surg Lasers Imaging. 2010;9:1-3.
- Zepeda-Romero LC, Oregon-Miranda AA, Lizarraga-Barrón DS, Gutiérrez-Camarena O, Meza-Anguiano A, Gutiérrez-Padilla JA. Early retinopathy of prematurity findings identified with fluorescein angiography. Graefes Arch Clin Exp Ophthalmol. 2013:251:2093-7.
- Bianciotto C, Shields CL, Iturralde JC, Sarici A, Jabbour P, Shields JA. Fluorescein angiographic findings after intra-arterial chemotherapy for retinoblastoma. Ophthalmology. 2012;119(4):843-849.
- Kim JW, Ngai LK, Sadda S, Murakami Y, Lee DK, Murphree AL. Retcam fluorescein angiography findings in eyes with advanced retinoblastoma. Br J Ophthalmol. 2014;98(12):1666-1671.
- Shields CL, Materin M, Shields JA. Panoramic imaging of the ocular fundus. Arch Ophthalmol. 2003;121(11):1603-1607.
- Manivannan A, Plskova J, Farrow A, Mckay S, Sharp PF, Forrester JV. Ultra-wide-field fluorescein angiography of the ocular fundus. Am J Ophthalmol. 2005;140(3):525-527.
- Friberg TR, Pandya A, Eller AW. Non-mydriatic panoramic fundus imaging using a non-contact scanning laser-based system. Ophthalmic Surg Lasers Imaging. 2003;34(6):488-497.
- Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123(2):244-252.
- Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis(®) noncontact ultra-widefield module versus the Optos(®) Optomap(®). Clin Ophthalmol. 2013;7:389-394.
- Shah AR, Abbey AM, Yonekawa Y, et al. Widefield fluorescein angiography in patients without peripheral disease: a study of normal peripheral findings. Retina. 2016;36(6):1087-1092.
- Kaneko Y, Moriyama M, Hirahara S, Ogura Y, Ohno-Matsui K. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55(3):1432-1439.
- Moriyama M, Ohno-Matsui K, Hayashi K, et al. Topographical analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011;118(8):1626-1637.
- Moriyama M, Ohno-Matsui K, Modegi T, et al. Quantitative analyses of high-resolution 3D MR images of highly myopic eyes to determine their shapes. Invest Ophthalmol Vis Sci. 2012;53(8):4510-4518.
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900-1901.
- Stefánsson E, Geirsdóttir A, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30(1):72-80.
- Madhusudhan S, Beare N. Wide-field fluorescein angiography in wet age-related macular degeneration. Scientific World J. 2014:536161.
- Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255(5):538-561.
- Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
- Shimizu K, Kobayashi Y, Muraoka K. Mid-peripheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88(7):601-612.
- Cardillo Piccolino F, Zingirian M, Mosci C. Classification of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1987;225(4):245-250.
- Early Treatment Diabetic Retinopathy Study Investigators. Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741-756.
- Patel RD, Messner LV, Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155(6):1083-1044.
- Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698.
- Kimble JA, Brandt BM, McGwin G, Jr. Clinical examination accurately locates capillary nonperfusion in diabetic retinopathy. Am J Ophthalmol. 2005;139(3):555-557.
- Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25(1-2):27-33.
- Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy: A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79(5):435-440.
- Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: Association with retinal photocoagulation. Diabetologia. 2000;43(11):1404-1407.
- Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133-141.
- Prasad PS, Oliver SC, Coffee RE, Hubschman JP, Schwartz SD. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology. 2010;117(4):780-784.
- Spaide RF. Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography. Retina. 2011;31(5):829-837.
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-516.
- Reeves GM, Kumar N, Beare NA, Pearce IA. Use of Staurenghi lens angiography in the management of posterior uveitis. Acta Ophthalmol. 2013;91(1):48-51.
- Tsui I, Kaines A, Schwartz S. Patterns of periphlebitis in intermediate uveitis using ultra wide field fluorescein angiography. Semin Ophthalmol. 2009;24(1):29-33.
- Karampelas M, Sim DA, Chu C, et al. Quantitative analysis of peripheral vasculitis, ischemia, and vascular leakage in uveitis using ultra-widefield fluorescein angiography. Am J Ophthalmol. 2015;159(6):1161-1168.
- Hong BK, Nazari Khanamiri H, Rao NA. Role of ultra-widefield fluorescein angiography in the management of uveitis. Can J Ophthalmol. 2013;48(6):489-493.
- Laws DE, Morton C, Weindling M, Clark D. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol. 1996;80(5):425-428.
- Rush R, Rush S, Ighani F, Irwin M, Naqvi M. The effects of comfort care on the pain response in preterm infants undergoing screening for retinopathy of prematurity. Retina. 2005;25(1):59-62.
- Fung TH, Yusuf IH, Xue K, Smith LM, Patel CK. Heidelberg Spectralis ultra-widefield fundus fluorescein angiography in infants. Am J Ophthalmol. 2015;159(1):78-84.
- Adams GG, Clark BJ, Fang S, Hill M. Retinal haemorrhages in an infant following RetCam screening for retinopathy of prematurity. Eye (Lond). 2004;18(6):652-653.
- Lim Z, Tehrani NN, Levin AV. Retinal haemorrhages in a preterm infant following screening examination for retinopathy of prematurity. Br J Ophthalmol. 2006;90(6):799-800.
- Kemper AR, Wallace DK, Quinn GE. Systematic review of digital imaging screening strategies for retinopathy of prematurity. Pediatrics. 2008;122(4):825-830.
- Fung THM, Yusuf IH, Smith LM, Brett J, Weston L, Patel CK. Outpatient ultra-widefield intravenous fundus fluorescein angiography in infants using the Optos P200MA scanning laser ophthalmoscope. Br J Ophthalmol. 2014;98(3):302-304.
- Fung THM, Muqit MMK, Mordant DJ, Smith LM, Patel CK. Non-contact ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014;132(1):108-110.
- Nicholson BP, Nigam Y, Miller D, et al. Comparison of wide-field fluorescein angiography and 9-field montage angiography in uveitis. Am J Ophthalmol. 2014;157(3):673-677.
- Azad R, Chandra P, Khan MA, Darswal A. Role of intravenous fluorescein angiography in early detection and regression of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2008;45(1):36-39.
- Patel CK, Fung TH, Muqit MM, Mordant DJ, Geh V. Non-contact ultra-widefield retinal imaging and fundus fluorescein angiography of an infant with incontinentia pigmenti without sedation in an ophthalmic office setting. J AAPOS. 2013;17(3):309-311.
- Do DV, Haller JA. Coats disease. In: Ryan SJ, Schachat AP, eds. Retina. Philadelphia, PA: Elsevier, 2006: 1417-1427.
- Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131(5):561-571.
- Kang KB, Wessel MM, Tong J, D’Amico DJ, Chan RV. Ultra-widefield imaging for the management of pediatric retinal diseases. J Pediatr Ophthalmol Strabismus. 2013;50(5):282-288.








