In using Iluvien (fluocinolone acetonide intravitreal implant 0.19 mg, Alimera Sciences) to treat diabetic macular edema (DME) in my practice, I’ve seen that it can have significant efficacy in eyes that aren’t responsive to other therapeutic approaches, including other steroids. For example, one of my patients, a pseudophakic 66-year-old female with a 24-year history of diabetes, is an Iluvien success story even though hers was a case of refractory DME.
Prior to receiving bilateral Iluvien implants, the patient developed proliferative diabetic retinopathy in both eyes, which was treated with panretinal photocoagulation. Subsequently, both eyes underwent vitrectomy to treat vitreous hemorrhage and moderate tractional retinal detachment. Both eyes remained stable without a need for additional surgery for approximately a year. In 2012, clinically significant DME was detected bilaterally. The edema wasn’t responsive to frequent injections of bevacizumab (Genentech), ranibizumab (Genentech), or aflibercept (Regeneron). It was also unresponsive to treatment with regional depot steroids, including triamcinolone acetonide injectable suspension and the dexamethasone intravitreal implant (Allergan).
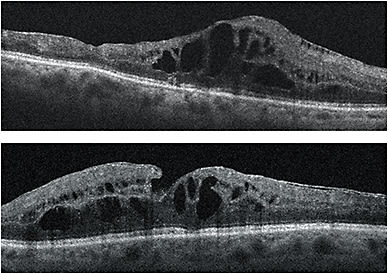
In 2015, because intraretinal fluid persisted in both eyes despite significant exposure to anti-VEGF agents and the dexamethasone intravitreal implant, I considered Iluvien to be the next logical step. The patient qualified for on-label use of Iluvien because she didn’t experience a significant increase in IOP with the dexamethasone implant; her IOP didn’t exceed 20 mmHg while that implant was in place. Within 2 months of bilateral Iluvien implantation, DME resolved in both eyes and visual acuity improved from 20/70 to 20/50 OU (Figures 1 and 2). In the first 10 months since implantation, her IOP has not exceeded 20 mmHg. The success of Iluvien in this case where other steroids had failed may be due to its unique stoichiometry. •