Update on Diagnosis and Treatment of CMV Retinitis
HAART has decreased the incidence, but a definitive cure for CMV retinitis remains elusive.
NICHOLAS J. BUTLER, MD • JENNIFER E. THORNE, MD, PhD
Nicholas Butler, MD, is assistant professor of ophthalmology at the Wilmer Eye Institute’s Division of Ocular Immunology. Jennifer E. Thorne, MD, PhD, is professor of ophthalmology and epidemiology and the director of the Division of Ocular Immunology at Wilmer. Neither author reports any financial interests in any of the products mentioned in this article. Dr. Thorne can be reached at jthorne@jhmi.edu. |
In the United States, exposure to cytomegalovirus (CMV) is ubiquitous; seroprevalence rises from nearly 60% in patients 6 years or older to greater than 90% in individuals 80 years or older.1
In people with intact immune systems, initial infection with CMV causes minimal, if any, symptoms, although it can induce a mononucleosis-like syndrome in some patients. Cell-mediated immunity controls the virus and prevents specific organ disease in all but a rare few.
However, in the setting of advanced immunosuppression, such as AIDS, organ transplantation with iatrogenic immunosuppression, autoimmune disease, or malignancy, susceptible individuals can develop specific end-organ CMV disease (eg, encephalitis, esophagitis, colitis, and retinitis), carrying a significant risk of morbidity and mortality.
Of these diseases, retinitis (Figures 1-4) occurs at a greatly increased rate compared to other CMV diseases, at least in AIDS patients,2 and it confers an increased risk of mortality.3

Figure 1. CMV retinitis displaying frosted branch angiitis.

Figure 2. CMV retinitis in a 69-year-old Caucasian man in chemotherapy-induced remission from non-Hodgkin lymphoma.

Figure 3. CMV retinitis in a 29-year-old African-American woman with HIV/AIDS and a CD4 count of 16.
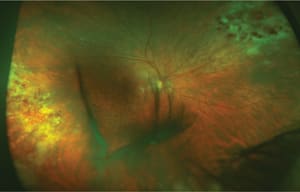
Figure 4. CMV retinitis in an 82-year-old Caucasian man with a history of sarcoidosis suppressed with mycophenolate mofetil (CellCept, Genentech) therapy.
A CHANGING LANDSCAPE
The development of highly active antiretroviral therapy (HAART) in 1996 changed the landscape considerably. The four-year cumulative incidence of CMV retinitis in AIDS patients with immune suppression (CD4+ T cell count <100 cells/μL) has decreased by 72% in the post-HAART era, as determined in the Longitudinal Ocular Complications of AIDS (LSOCA) study.4
This cohort study, which has followed AIDS-defined patients for 15 years, likely included patients at relatively higher risk for developing ocular opportunistic infections than the general HIV population. In fact, the incidence of CMV retinitis has likely declined by 90% in many academic centers.
Nonetheless, patients still develop this sight-threatening disease, likely in the setting of diagnostic delay, noncompliance, or nonresponse to HAART, and the single most important risk factor for the development of CMV retinitis in AIDS patients remains a CD4+ T cell count <50 cells/μL.4
Because patients with AIDS live longer with HAART-induced immune recovery, a smaller percentage of those living with HIV remain at increased risk for developing CMV retinitis. As such, the proportion of non–AIDS-related CMV retinitis cases, those immunosuppressed by other means, and even cases in some otherwise healthy individuals might be increasing.
This article reviews the current CMV retinitis literature, with attention directed toward recent advances in the diagnosis and treatment of this disease.
DIAGNOSIS
Ophthalmoscopic Findings
Clinicians generally make the diagnosis of CMV based on clinical grounds alone, in the setting of a supportive history of an immune-compromised state (HIV/AIDS, iatrogenic, malignancy, or other).
Retinal lesions commonly appear peripherally in a peri-vascular distribution as a creamy white infiltrate, with a more granular border comprised of smaller satellite lesions. Less obviously involved spaces of “clear” retina separate the granular foci.
However, with time, these areas progress to active retinitis and scarring, suggesting involvement with the virus from the beginning. The active border generally advances posteriorly, at a rate of 250-350 μm/week, leaving a swath of scarred and necrotic retina with mottled pigmentation of the RPE in its wake. Lesions presenting more posteriorly can involve the retinal vessels and cause retinal hemorrhages, giving rise to the term “pizza pie” retinitis.
The clinical appearance of newly diagnosed CMV retinitis among highly immunosuppressed patients with AIDS has not changed appreciably from the pre-HAART era to the HAART era.5 However, the rates of developing retinitis and its attendant complications, such as retinal detachment6 and loss of visual acuity7 and visual field,8 have dramatically improved with HAART.
Clinical Findings of CMV Retinitis In Non–HIV-positive Patients
Because patients with HIV/AIDS live longer with HAART-induced immune recovery, the proportion of non–HIV/AIDS-related CMV retinitis might be rising, and the clinical features can be somewhat different in this population.
In elderly immunocompetent patients (other than the natural waning of immunity and increased prevalence of typical comorbidities that come with age), CMV retinitis appears to have an increased association with retinal arteriolar occlusions, even in the setting of minimal retinitis.9,10
Other investigators have echoed these findings. In patients with limited immune dysfunction (due to old age, diabetes, or noncytotoxic immunosuppression), CMV retinitis can present with typical peripheral, granular, and slowly progressive retinitis and an atypical panretinal occlusive vasculitis, mimicking acute retinal necrosis in its retinal vascular appearance only.11
Eighty percent of patients in this small series went on to develop neovascularization, due to the extent of induced retinal ischemia. In a series of 18 mostly younger Thai patients (mean age 49 years) without HIV, the majority of whom were immunosuppressed by other causes, retinal vasculitis appeared in 73%. Among this group, 81% primarily had arteritis, in contrast to CMV retinitis patients with HIV, in whom periphlebitis was more typical.12
Ocular Imaging
Determining progression of retinitis, which involves documentation of nearly imperceptible border movement or the development of a new focus of retinitis, is optimally aided with serial fundus photographs. This becomes particularly important in deciding upon response to treatment.
Recently, investigators have demonstrated that hyperautofluorescence in areas of CMV retinitis bordering activity on fundus autofluorescence imaging may be a useful adjunct in differentiating between active and inactive retinitis when the clinical exam and photos are equivocal.13
Additionally, optical coherence tomography has revealed subhyaloid, presumed inflammatory, precipitates in a small series of patients with active CMV retinitis.14 Whether or not these examination and imaging findings add to our understanding of the clinical features of this disease in a meaningful way (ie, diagnostic relevance or response to treatment) remains to be determined.
Biochemical Testing
In cases of diagnostic dilemma, especially in the absence of an identifiable source of host immunosuppression, polymerase chain reaction (PCR) of aqueous or vitreous samples can amplify CMV DNA and secure a diagnosis.15
In most clinical centers that treat cases of CMV retinitis frequently, PCR of ocular fluid is often performed at the initial consultation, because rapid differentiation among retinal infections associated with herpes simplex virus, varicella zoster virus, and CMV has important treatment implications.
More recently, a loop-mediated isothermal amplification (LAMP) assay demonstrated 100% concordance with PCR in detecting CMV DNA in vitreous samples of patients with CMV retinitis at a fraction of the cost, potentially increasing the diagnostic capabilities of clinicians in resource-limited settings.16
Screening
Most uveitis and retina specialists who regularly follow patients with HIV screen individuals with CD4+ T cell counts <50 cells/μL, using dilated fundus exams every three months.
As the cost of medicine increases, and in resource-limited settings in which the burden of CMV retinitis exceeds the supply of qualified physicians, the use of telemedicine screening for CMV retinitis with fundus photography has increased, demonstrating validity.17,18
In patients undergoing allogenic hematopoietic stem cell transplantation (HSCT) after conditioning with an alemtuzumab (Campath, Genzyme, Cambridge, MA)-based regimen, the frequency of CMV retinitis can approach 24%.19 Regular screening for retinitis in this population is also warranted.
Similarly, post-HSCT patients with a significant CMV viral load (peak CMV DNA level >7.64 x 104 copies/mL) are at increased risk of developing CMV retinitis (hazard ratio, 25.0; 95% confidence interval, 3.0-210.8), so physicians should monitor patients in this subgroup closely.20
Pitfalls of Delayed Diagnosis
Diagnostic delay can increase the risk of vision loss, both directly, through more fulminant retinitis and a greater likelihood of foveal or optic nerve involvement,21 and indirectly, because the burden of immune recovery uveitis (IRU) and its sequelae increases with the severity of CMV retinitis.22
Further, in elderly, otherwise healthy patients with CMV retinitis, delayed diagnosis has contributed to associated retinal arteriolar occlusions, with sometimes disastrous visual outcomes.9
MANAGEMENT
Because no currently available agent is virucidal, the goal of therapy generally is to arrest viral replication/assembly until the host’s immune system has recovered sufficiently to assume this function.
In the setting of HIV/AIDS, initiation of HAART is the most critical step in planning for long-term suppression of CMV retinitis. However, some infectious disease specialists favor a delay in HAART, with the hope of reducing the development of immune-recovery syndromes.23
Research has demonstrated the potential benefits of this strategy in the eye, including a lowered incidence of IRU.24 In the event that immune system recovery is unexpected (ie, transplant recipients requiring lifelong immunosuppression), the physician should provide indefinite virostatic treatment.
Cytomegalovirus DNA can be recovered from dormant CMV retinitis lesions. However, histopathology has revealed dysfunction in the assembly of intact virions in patients on chronic therapy.25
SYSTEMIC THERAPY
Although CMV retinitis can present as an isolated infection, often initially localized in only one eye, the disease itself is part of a systemic infection with implications for the fellow eye and other organs. As such, patients generally always receive systemic therapy, with or without concurrent local treatment.
The FDA has approved three intravenous drugs for the treatment of CMV retinitis: oral or intravenous ganciclovir (Cytovene, Roche, Nutley, NJ); intravenous foscarnet (Foscavir, AstraZeneca, Wilmington, DE); and intravenous cidofovir (Vistide, Gilead, Foster City, CA). Systemic therapies generally commence at higher induction dosages for two to three weeks, followed by lower maintenance doses to prevent relapse of the retinitis.
All three currently available intravenous drugs employ analogous mechanisms of action, specifically selective inhibition of viral DNA polymerase.26 Ganciclovir appeared first in 1989, followed by foscarnet in 1991.
A large randomized trial revealed no difference between intravenous ganciclovir and intravenous foscarnet with regard to the rate of retinitis progression. However, the Policy and Data Monitoring Board terminated the study due to a 79% increase in mortality in the ganciclovir arm.
The authors speculated that the survival difference stemmed from the intrinsic anti-HIV effects of foscarnet or the increased rates of concomitant zidovudine use in the foscarnet group (due to synergistic myelosuppressive effects of zidovudine with ganciclovir). However, more extensive analyses failed to demonstrate this latter hypothesis conclusively.
At any rate, this study occurred prior to the HAART era, and the authors presciently advised caution in applying their data widely, because an explosion of antiretroviral drugs occurred shortly after this study’s 1992 publication date.27
Newer Formulations, Including Oral
Because comparative trials have demonstrated similar efficacy for all systemic medications regarding time to progression of retinitis, the use of oral valganciclovir (Valcyte, Roche), an L-valyl ester prodrug of ganciclovir, has largely surpassed the use of intravenous formulations.28
Oral valganciclovir obviates much of the inconvenience and risk associated with intravenous therapy, although cost and myelosuppression remain issues. The drug achieves a bioavailability of 60%, comparable to intravenous ganciclovir and far greater than that of oral ganciclovir (5%).29
Several newer antiviral agents are in various stages of development, from preclinical experiments to phase 2 clinical trials. Cidofovir esters were 1,000 times more potent against human and murine CMV in in vitro studies, with improved oral bioavailability (88-97%) and less renal toxicity.
Other agents, such as maribavir (GlaxoSmithKline, Philadelphia, PA), BAY 38-4766 (Bayer, Pittsburgh, PA), and AIC246 (AiCuris, Wuppertal, Germany), inhibit viral activity through pathways other than the inhibition of viral DNA polymerase. As a result, they decrease the chances of cross-resistance with the currently FDA-approved medications.29
Intravitreal Therapy
Patients commonly receive intravitreal injections of either ganciclovir or foscarnet, with or without systemic medication, to control sight-threatening retinitis (zone 1 disease) or to help bridge to an alternative systemic therapy when a suspicion of resistance arises.
Induction dosing with intravitreal medications requires injections two to three times weekly, while once weekly generally is sufficient for maintenance. In developing countries, where cost can be a major barrier to treatment, intravitreal strategies alone are being increasingly employed, demonstrating comparable efficacy in terms of control of retinitis at only 11.7% and 11.1% of the cost of sustained-release implants and systemic therapy, respectively.30
We should reiterate, however, that local therapy alone was associated with a higher risk of other end organ involvement (including the fellow eye) and death in the pre-HAART era.31–33 This association has continued into the current HAART era.28,34
Further, the latest data from LSOCA have indicated that patients treated with intravitreal therapy alone, compared to those treated with systemic regimens (with or without intravitreal therapy) or the ganciclovir implant, might have worse ocular outcomes, in terms of final VA, progression of retinitis, and loss of visual field, even after adjusting for known confounders.34
The authors have highlighted, however, the relatively small sample size of patients with local therapy alone and the potential for unrecognized, and hence unmeasured, bias.
Data From Outside the HIV/AIDS Cohort
Much of the data in support of intravitreal approaches for CMV retinitis have come from adult patients with AIDS. However, more recently, reports have emerged of the safety and efficacy of intravitreal ganciclovir for CMV retinitis in neonates with congenital infection35,36 and in patients post-HSCT.37
In some instances of temporary iatrogenic immunosuppression (ie, patients in remission post-treatment for autoimmune disease or cancer), ongoing immunosuppression can stem from systemic treatment of CMV retinitis, because ganciclovir and valganciclovir can be profoundly myelosuppressive.
Stopping systemic anti-CMV therapy and initiating intravitreal ganciclovir or foscarnet can induce sufficient immune recovery to provide long-term drug-free remission of CMV retinitis.38
However, if immune recovery does not or cannot occur, the rates of relapse of infection with ongoing intravitreal injections are comparable to those with systemically administered regimens, even with aggressive therapy.39,40
Ganciclovir Implant
In 1996, the FDA approved a sustained-release, intra-ocular ganciclovir implant (Vitrasert, Bausch + Lomb, Rochester, NY). In the pre-HAART era, this implant demonstrated superiority over intravenous ganciclovir in terms of median time to the progression of retinitis (221 days for the 1 μg/hour implant vs 71 days for intravenous ganciclovir).31
As expected, patients treated with the implant alone developed CMV disease outside of the treated eye at higher rates, and oral or intravenous ganciclovir significantly reduced this risk.31,32
Complication rates associated with the ganciclovir implant, most commonly for cataract, vitreous hemorrhage, and retinal detachment, have remained relatively high (0.19/eye-year). However, severe vision loss is infrequent (0.005/eye-year for ≥6 lines of vision loss).41
Beyond the known benefit that immune recovery has on mitigating the complications of CMV retinitis, it seems that immune recovery may similarly decrease the rates of complications associated with the implant.41
Although the ganciclovir implant, coupled with oral ganciclovir, offers the greatest protection against progression of retinitis demonstrated to date,32 it is no longer on the market due to unfavorable economics with the declining incidence of CMV retinitis. The effectiveness and ease of use of oral valganciclovir likely hastened the departure of the implant as well.
Immune Recovery
A recovered immune system can control CMV retinitis in the absence of ongoing prophylactic therapy. The Department of Health and Human Services currently recommends that HIV patients with CD4 counts of >100 cells/μL for at least three to six months discontinue anti-CMV therapy41; data from LSOCA support adherence to these recommendations.43
Physicians should still follow these patients with dilated exams every three months, because not all immune-recovered patients will suppress the retinitis without therapy, and the risk of relapse persists for five years at minimum.28
While HAART-induced immune recovery may indefinitely control the infection, it has also been associated with the development of IRU in nearly 20% of patients with CMV retinitis.22,28
Many of these patients are at increased risk for vision loss, mainly from CME, which may be responsive to local or systemic corticosteroids but often recrudesces. The fluocinolone acetonide implant (Retisert, Bausch + Lomb) appears to control IRU and CME in a few eyes, but long-term data to ascertain the risk of retinitis reactivation have been limited.44
Antiviral Resistance
If immune recovery does not occur (or is not possible), relapse of retinitis is the norm, even in patients with strict adherence to suppressive therapy. Early relapse often relates to insufficient intraocular concentration of drug, while late relapse typically stems from emergent resistance.28
Mutations in the CMV UL97 gene, a viral phosphotransferase necessary for ganciclovir activation, confer low-level resistance to ganciclovir, while mutations in both the CMV UL97 and CMV UL54 genes lead to high-level ganciclovir resistance. Researchers have theorized that low-level ganciclovir-resistant CMV should respond to cidofovir, but high-level resistance mandates foscarnet therapy.28
In practice, physicians rarely prescribe cidofovir due to high rates of associated renal toxicity and hypotonous uveitis. Beyond the implications for the eye with CMV retinitis, demonstration of resistant CMV in the blood confers a 65% increase in mortality in patients with AIDS and CMV retinitis.45
Coinfection by multiple strains of CMV may exist in any one patient. Due to variance in selective pressures among differing body sites, it is possible to demonstrate peripheral blood resistance to ganciclovir in the setting of ocular susceptibility46; so when assessing for resistance to ganciclovir in cases of CMV retinitis, ocular fluid analysis with PCR is informative.47
Treatment Options in Resistance
Until newer antivirals reach the level of commercial availability, treatment of CMV retinitis in the setting of drug resistance remains a particular challenge. Oral leflunomide (Arava, Sanofi-Aventis, Bridgewater, NJ), an immunosuppressive agent with anti-CMV activity, has demonstrated efficacy in transplant patients with systemic CMV infection48 and also in multi–drug-resistant CMV retinitis.49
An additional advantage of leflunomide includes a significant cost reduction, compared to intravenous ganciclovir or oral valganciclovir. However, due to variability in half-life of the active metabolite, serum level monitoring is mandatory (range from 25 ng/mL to 80 μg/mL).48
Given the cost advantage and well-demonstrated inferiority of intravitreal injections alone for CMV retinitis, it may be beneficial to add oral leflunomide to intravitreal therapy in resource-limited locales. We should note, however, that evidence in support of this combination has been lacking.
Response to Treatment
Monitoring for response to treatment with frequent clinical examinations, supplemented with fundus photography, is critical, as randomized CMV retinitis treatment trials previously advocated.50,51
Because the disease may progress very slowly, border movement may evade detection if the physician relies on fundus examinations and memory alone.
CONCLUSION
Despite the manifest benefits of HAART on incidence and severity, CMV retinitis remains the most common ocular opportunistic infection in patients with AIDS.7,52 Patients with a CD4+ T cell count <50 continue to be at increased risk of CMV retinitis, and frequent screening in this population is essential to detect the disease before it becomes sight-threatening.
Initiation of HAART has direct and indirect benefits, although the optimal timing of initiation, with regard to the risk of immune reconstitution syndromes, remains under investigation.
Further, among non-HIV infected individuals, we may witness an increasing number of CMV retinitis cases in otherwise immunosuppressed people, because more clinicians use immunosuppressive therapy for auto-immune diseases and malignancies.
As a group, our collective education during the AIDS epidemic regarding the clinical features of CMV retinitis may contribute to an increased ability to recognize and diagnose the disease in atypical settings, such as the immunocompetent host. However, the disease in patients without AIDS may be phenotypically distinct, with a greater likelihood of sight-threatening retinal arteriolar occlusions.
Newer anti-CMV therapeutics, with better safety profiles and increased efficacy, are in various stages of development. Their emergence on the market is critical in addressing the high rates of reactivation and antiviral resistance that occur with long-term virostatic therapy for CMV retinitis.
In the developing world, where resources may limit the use of oral valganciclovir or intravenous ganciclovir, intra-vitreal injections alone likely confer a worse prognosis, in terms of other end-organ damage and overall mortality. Adjuvant agents, such as oral leflunomide or oral ganciclovir, may be cost-effective options in mitigating the systemic consequences of this disease in such locales. RP
REFERENCES
1. Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143-1151.
2. Gallant JE, Moore RD, Richman DD, et al. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis. 1992;166:1223-1227.
3. Holland GN, Sison RF, Jatulis DE, et al. Survival of patients with the acquired immune deficiency syndrome after development of cytomegalovirus retinopathy. UCLA CMV Retinopathy Study Group. Ophthalmology. 1990;97:204-211.
4. Sugar EA, Jabs DA, Ahuja A, et al. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012;153:1016-1024.
5. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787-793.
6. Holbrook JT, Jabs DA, Weinberg DV, et al. Studies of Ocular Complications of AIDS (SOCA) Research Group. Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy. Arch Ophthalmol. 2003;121:99-107.
7. Thorne JE, Jabs DA, Kempen JH, et al. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1432-1440.
8. Thorne JE, Van Natta ML, Jabs DA, et al. Studies of Ocular Complications of AIDS Research Group. Visual field loss in patients with cytomegalovirus retinitis. Ophthalmology. 2011;118:895-901.
9. Davis JL, Haft P, Hartley K. Retinal arteriolar occlusions due to cytomegalovirus retinitis in elderly patients without HIV. J Ophthalmic Inflamm Infect. 2013;3:17.
10. Gupta S, Vemulakonda GA, Suhler EB, et al. Cytomegalovirus retinitis in the absence of AIDS. Can J Ophthalmol. 2013;48:126-129.
11. Schneider EW, Elner SG, van Kuijk FJ, et al. Chronic retinal necrosis: cytomegalovirus necrotizing retinitis associated with panretinal vasculopathy in non-HIV patients. Retina. 2013;33:1791-1799.
12. Pathanapitoon K, Tesavibul N, Choopong P, et al. Clinical manifestations of cytomegalovirus-associated posterior uveitis and panuveitis in patients without human immunodeficiency virus infection. JAMA Ophthalmol. 2013;131:638-645.
13. Yeh S, Forooghian F, Faia LJ, et al. Fundus autofluorescence changes in cytomegalovirus retinitis. Retina. 2010;30:42-50.
14. Goldhardt R, Gregori NZ, Albini T, et al. Posterior subhyaloid precipitates in cytomegalovirus retinitis. J Ophthalmic Inflamm Infect. 2012;2:41-45.
15. Tran TH, Rozenberg F, Cassoux N, et al. Polymerase chain reaction analysis of aqueous humour samples in necrotizing retinitis. Br J Ophthalmol. 2003;87:79-83.
16. Reddy AK, Balne PK, Reddy RK, et al. Development and evaluation of loop-mediated isothermal amplification assay for rapid and inexpensive detection of cytomegalovirus DNA in vitreous specimens from suspected cases of viral retinitis. J Clin Microbiol. 2010;48:2050-2052.
17. Ausayakhun S, Skalet AH, Jirawison C, et al. Accuracy and reliability of telemedicine for diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 2011;152:1053-1058.
18. Shah JM, Leo SW, Pan JC, et al. Telemedicine screening for cytomegalovirus retinitis using digital fundus photography. Telemed J E Health. 2013;19:627-631.
19. Song WK, Min YH, Kim YR, et al. Cytomegalovirus retinitis after hematopoietic stem cell transplantation with alemtuzumab. Ophthalmology. 2008;115:1766-1770.
20. Jeon S, Lee WK, Lee Y, et al. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology. 2012;119:1892-1898.
21. Ausayakhun S, Keenan JD, Ausayakhun S, et al. Clinical features of newly diagnosed cytomegalovirus retinitis in northern Thailand. Am J Ophthalmol. 2012;153:923-931.
22. Kempen JH, Min YI, Freeman WR, et al. Studies of Ocular Complications of AIDS Research Group. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113:684-694.
23. Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol. 2010;4:285-299.
24. Ortega-Larrocea G, Espinosa E, Reyes-Terán G. Lower incidence and severity of cytomegalovirus-associated immune recovery uveitis in HIV-infected patients with delayed highly active antiretroviral therapy. AIDS. 2005;19:735-738.
25. Pepose JS, Holland GN, Nestor MS, et al. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472-484.
26. Huynh N, Daniels AB, Kohanim S, et al. Medical treatment for cytomegalovirus retinitis. Int Ophthalmol Clin. 2011;51:93-103.
27. Studies of ocular complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for CMV retinitis. N Engl J Med. 1992;326:213-220.
28. Jabs DA. Cytomegalovirus retinitis and acquired immunodeficiency syndrome—bench to bedside: LXVII Edward Jackson memorial lecture. Am J Ophthalmol. 2011;151:198-216.
29. Vadlapudi AD, Vadlapatla RK, Mitra AK. Current and emerging antiviral for the treatment of cytomegalovirus (CMV) retinitis: an update on recent patents. Recent Pat Antiinfect Drug Discov. 2012;7:8-18.
30. Teoh SC, Ou X, Lim TH. Intravitreal ganciclovir maintenance injection for cytomegalovirus retinitis: efficacy of a low-volume, intermediate-dose regimen. Ophthalmology. 2012;119:588-595.
31. Musch DC, Martin DF, Gordon JF, et al. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337:83-90.
32. Martin DF, Kuppermann BD, Wolitz RA, et al. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340:1063-1070.
33. Kempen JH, Jabs DA, Wilson LA, et al. Mortality risk for patients with cytomegalovirus retinitis and acquired immune deficiency syndrome. Clin Infect Dis. 2003;37:1365-1373.
34. Jabs DA, Ahuja A, Van Natta M, et al. Studies of the Ocular Complications of AIDS Research Group. Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2013;120:1262-1270.
35. Lalezary M, Recchia FM, Kim SJ. Treatment of congenital cytomegalovirus retinitis with intravitreous ganciclovir. Arch Ophthalmol. 2012;130:525-527.
36. Oschman A, Murthy V, Kollipara R, et al. Intravitreal ganciclovir for neonatal cytomegalovirus-associated retinitis: a case report. J Perinatol. 2013;33:329-331.
37. Miao H, Tao Y, Jiang YR, et al. Multiple intravitreal injections of ganciclovir for cytomegalovirus retinitis after stem-cell transplantation. Graefes Arch Clin Exp Ophthalmol. 2013;251:1829-1833.
38. Langner-Wegscheider BJ, ten Dam-van Loon N, Mura M, et al. Intravitreal ganciclovir in the management of non-AIDS-related human cytomegalovirus retinitis. Can J Ophthalmol. 2010;45:157-160.
39. Diaz-Llopis M, España E, Muñoz G, et al. High dose intravitreal foscarnet in the treatment of cytomegalovirus retinitis in AIDS. Br J Ophthalmol. 1994;78:120-124.
40. Young S, Morlet N, Besen G, et al. High-dose (2000-μg) intravitreous ganciclovir in the treatment of cytomegalovirus retinitis. Ophthalmology. 1998;105:1404-1410.
41. Oktavec KC, Nolan K, Brown DM, et al. Clinical outcomes in patients with cytomegalovirus retinitis treated with ganciclovir implant. Am J Ophthalmol. 2012;153:728-733.
42. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Morb Mortal Wkly Rep. 2009;58(RR-4):55.
43. Holbrook JT, Colvin R, van Natta ML, et al. Studies of Ocular Complications of AIDS (SOCA) Research Group. Evaluation of the United States public health service guidelines for discontinuation of anticytomegalovirus therapy after immune recovery in patients with cytomegalovirus retinitis. Am J Ophthalmol. 2011;152:628-637.
44. Hu J, Coassin M, Stewart JM. Fluocinolone acetonide implant (Retisert) for chronic cystoid macular edema in two patients with AIDS and a history of cytomegalovirus retinitis. Ocul Immunol Inflamm. 2011;19:206-209.
45. Jabs DA, Martin BK, Forman MS, et al. Mortality associated with resistant cytomegalovirus among patients with cytomegalovirus retinitis and AIDS. Ophthalmology. 2010;117:128-132.
46. Bakshi NK, Fahle GA, Sereti I, et al. Cytomegalovirus retinitis successfully treated with ganciclovir implant in a patient with blood ganciclovir resistance and ocular ganciclovir sensitivity. Eye (Lond). 2012;26:759-760.
47. Yeh S, Fahle G, Forooghian F, et al. Polymerase chain reaction-based ganciclovir resistance testing of ocular fluids for cytomegalovirus retinitis. Arch Ophthalmol. 2012;130:113-115.
48. John GT, Manivannan J, Chandy S, et al. A prospective evaluation of leflunomide therapy for cytomegalovirus disease in renal transplant recipients. Transplant Proc. 2005;37:4303-4305.
49. Dunn JH, Weinberg A, Chan LK, et al. Long-term suppression of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. JAMA Ophthalmol. 2013;131:958-960.
50. Musch DC, Martin DF, Gordon JF, et al. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337:83-90.
51. Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346:1119-1126.
52. Jabs DA, Ahuja A, Van Natta M, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152-2161.








