SUBSPECIALTY NEWS
Self-injected DME Drug in Trial
Aerpio tests subcutaneous delivery.
BY JERRY HELZNER, SENIOR EDITOR
■ Aerpio Therapeutics, a Cincinnati-based clinical-stage biopharmaceutical company targeting the Tie2 pathway, is conducting a phase 1b/2a trial for treatment of DME using a novel human protein tyrosine phosphatase beta (HPTPB) inhibitor the company has designated as AKB-9778. In a departure from the typical delivery methods for ophthalmic drugs, AKB-9778 is designed to be self-administered through subcutaneous injection daily over a four-week period.
EASY TO ADMINISTER
“Because our patients are all diabetics, most of them have experience doing their own subcutaneous insulin injections and they were easily able to self-administer after very brief training,” says Joseph Gardner, PhD, CEO of Aerpio.
Dr. Gardner says one of the reasons Aerpio selected DME as the disease target is because diabetic patients would be able to handle the subcutaneous self-administration.
“But that was not the primary reason DME was selected,” he notes. “The drug is more than an eye drug. We believe it can be beneficial in combating other systemic diabetic complications. It could also be complementary to anti-VEGF therapy and be used in combination with an anti-VEGF agent.”
RECEPTOR ACTIVATION IS KEY
The company believes that activating the Tie2 receptor on vascular endothelial cells promotes vascular stability, preventing abnormal blood vessel growth and vascular leakage.
“Recent preclinical and clinical data indicate that decreased signaling through the Tie2 pathway plays an important role in the development of diabetic retinopathy and possibly other diabetic vascular comorbidities, such as nephropathy and neuropathy,” says Kevin Peters, MD, Aerpio’s chief scientific officer and vice president of research and development. “Based on our preclinical data showing that, through Tie2 activation, AKB-9778 reduces retinal edema and neovascularization in multiple models of retinopathy, as well as data from our phase 1 study in 2012, we are optimistic that AKB-9778 could represent an important advancement in the treatment of DME.”
ENDPOINTS OUTLINED
The 28-day phase 1b/2a ascending dose study is designed to evaluate the safety and efficacy of AKB-9778 in patients with DME. The 24-patient study is being conducted at six sites throughout the United States. The primary endpoint is safety, with secondary efficacy endpoints based on decreased retinal thickness measured by OCT, improved visual acuity, and selected biomarkers.
Tie2 is a receptor tyrosine kinase expressed on vascular endothelial cells, which plays a key role in stabilizing blood vessels. AKB-9778 works by inhibiting the HPTPB enzyme, a negative regulator of the Tie2 receptor. In a phase 1 healthy volunteer study, Aerpio says AKB-9778 was well tolerated through the predicted efficacious dose range, with evidence of on-target pharmacology. Data from the phase 1b/2a study will be presented at the AAO meeting this fall.
| IN BRIEF |
|---|
|
■ B + L launches new PreserVision formulation. Bausch + Lomb is introducing a new PreserVision eye vitamin and mineral supplement whose formulation exactly matches the updated formula based on the latest clinical evidence from the National Eye Institute (NEI) Age-Related Eye Disease Study 2 (AREDS2) study. The daily dose (two soft gels) of new PreserVision AREDS 2 Formula provides the same levels of all six clinically proven nutrients as the NEI supported formula: vitamin C (500 mg), vitamin E (400 IU), lutein (10 mg)/zeaxanthin (2 mg), zinc (80 mg zinc oxide), and copper (2mg cupric oxide). ■ EU panel backs Eylea for new indication. Regeneron Pharmaceuticals said Eylea (aflibercept) has been recommended for approval by the European Committee for Medicinal Products for Human Use for treatment of macular edema secondary to CRVO. The decision of the European Commission is expected in the second half of 2013. Eylea was approved in the United States for the treatment of wet AMD in November 2011 and for macular edema following CRVO in September 2012. Eylea has also been approved in Europe, Japan, Australia and in several other countries for the treatment of wet AMD. |
Ophthotech Files Stock Offering
Its combination wet AMD therapy shows promise.
■ Ophthotech Corp., which has released positive data relating to its phase 2b clinical trial for its Fovista (anti-PDGF) and ranibizumab combination therapy for treating wet AMD, will soon become a public company after filing a stock offering with the SEC. Ophthotech shares will trade on the NASDAQ stock exchange under the symbol OPHT.

Ophthotech has raised $175 million from its venture capital partners to conduct a 1,900-patient phase 3 clinical trial involving more than 200 sites worldwide. Though the Fovista plus anti-VEGF combination therapy requires two injections, the phase 2b trial demonstrated twice the duration of response as ranibizumab (Lucentis, Genentech) monotherapy. No serious safety issues were reported in the phase 2b study.
Ophthotech is headed by chairman and CEO David Guyer, MD, and board vice-chairman Samir Patel, MD, who is overseeing clinical development of the combination therapy. Both men were also key figures in forming Eyetech Corporation for the original funding and development of Macugen (pegaptanib sodium, Valeant Pharmaceuticals), the first anti-VEGF drug approved for treatment of wet AMD.
Although soon eclipsed by the advent of ranibizumab, the development of Macugen was ground-breaking in the fact that it demonstrated that wet AMD progression could be at least halted or limited by regular intravitreal injections.
Eyetech also became a publicly traded company and was eventually sold to OSI Pharmaceuticals, which had little success with Macugen once the FDA approved ranibizumab.
Eyedrop Will Be Studied for RVO Indication
Squalamine also in wet AMD trial.
■ John J. Wroblewski, MD, senior partner at Cumberland Valley Retina Consultants, Hagerstown, MD, has initiated an investigator-sponsored 20-patient study that will use the eyedrop formulation of the aminosterol squalamine to treat macular edema secondary to retinal vein occlusion.
Dr. Wroblewski has also joined the scientific advisory board of Ohr Pharmaceuticals, which is conducting a large phase 2 clinical trial for the use of squalamine eyedrops to treat wet AMD. Squalamine appears naturally in the dogfish shark but has been synthesized for therapeutic use.
“The broad clinical potential of Squalamine has encouraged me to initiate a trial to look at this interesting drug candidate in retinal vein occlusion,” says Dr. Wroblewski. “Squalamine is known to inhibit multiple protein growth factors and it is now well established that agents with anti-VEGF properties have benefit for patients with RVO and improve visual acuity. The eyedrop formulation of squalamine may provide a non-invasive approach that is more convenient for patients and physicians.”
Patients in the study will receive squalamine eyedrops for the first two weeks of treatment, at which point they will then receive two successive injections of ranibizumab (Lucentis, Genentech) four weeks apart while continuing treatment with the eyedrops. Patients will subsequently be randomized 1:1 at week 10 to either continue administering squalamine eyedrops or discontinue drops for the remainder of the 38-week study. Rescue injections of Lucentis will be administered as needed though week 38.
The primary and secondary endpoints include visual acuity parameters, need for rescue retreatments, retinal thickness, vascular leakage, and change in area of non-perfusion.
Eylea is Effective in DME Trials
Regeneron will seek early approval.
■ Regeneron Pharmaceuticals and Bayer HealthCare said one-year data from two phase 3 trials of Eylea (aflibercept) for the treatment of DME were positive and that both companies would seek early approval of Eylea for the DME indication — Regeneron in the United States and Bayer in Europe.
Based on the one-year data and discussions with the FDA, Regeneron now expects to submit an application for US marketing approval for the treatment of DME this year, approximately one year ahead of the previously announced timeline.
TRIALS HAVE THREE ARMS
Both the VIVID and VISTA DME studies are ongoing and consist of three arms: Eylea 2 mg dosed monthly, Eylea 2 mg dosed every two months after five initial monthly injections, and a comparator arm of laser photocoagulation.
At 52 weeks, both Eylea arms achieved the primary endpoint of a significantly greater improvement in BCVA from baseline compared to laser photocoagulation. Both Eylea treatment arms demonstrated similar improvements in BCVA.
In the VIVID trial, after one year patients receiving Eylea 2 mg monthly had a mean change from baseline in BCVA of 10.5 letters, while those with every other month dosing has a mean gain of 10.7 letters. In the VISTA trial, after one year patients receiving Eylea 2 mg monthly had a mean increase in BCVA of 12.5 letters and those receiving every other month dosing had a mean BCVA improvement of 10.7 letters. BCVA gains at 52 weeks for the laser photocoagulation cohorts were minimal, averaging 0.2 letters in the VISTA trial and 1.2 letters in the VIVID study.
Adverse events (AEs) were typical of those seen in other studies in patients with diabetes receiving intravitreal anti-VEGF therapy. The most frequent ocular treatment emergent AEs observed in the VIVID and VISTA trials included conjunctival hemorrhage, eye pain, and vitreous floaters. The most frequent non-ocular AEs included hypertension and nasopharyngitis, which occurred with similar frequency in the treatment groups and the laser control group.
BOTH STUDIES TO CONTINUE
Full one-year data from the VIVID and VISTA trials will be presented at upcoming medical conferences. Both trials are planned to continue for up to 148 weeks.
Regeneron said US Eylea second-quarter sales gained approximately 70% to $330 million from $194 million in the year-ago quarter. The company now sees Eylea full-year US sales of $1.3 billion to $1.35 billion.
| IN BRIEF |
|---|
|
■ Lucentis sales increase on new FDA approvals. Roche recently reported that sales of Lucentis (ranibizumab) were up 9% in the first half of 2013 compared to the first six months of 2012. The company attributed the rise in revenues to the decision of the FDA last year to approve Lucentis as a treatment for DME and to the FDA’s approval of a 2013 label change for Lucentis that expands the suggested regimen to less-than-monthly dosing. ■ First European sales of Iluvien. Alimera Sciences has reported the first sales of its Iluvien sustained-release implant for the treatment of DME following approval in several European countries. Initial sales were in the UK and Germany and amounted to $179,000 in the second quarter. “We began generating revenue from Iluvien in the second quarter of 2013 in Germany and the United Kingdom and continue to make progress in gaining market access in Germany, the United Kingdom and France,” said Dan Myers, Alimera’s president and CEO. In addition, the Transparency Commission of the French National Health Authority has issued a favorable opinion for the reimbursement and hospital listing of Iluvien by the French National Health Insurance. RP |
| RETINAL PHYSICIAN READER POLL |
|---|
|
What is your primary medical therapy for wet AMD?
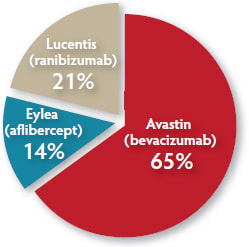
Based on 100 responses. Answer our new question: How has AREDS2 influenced your use of nutritional supplements for patients with wet AMD? www.retinalphysician.com |








