CURRENT CONCEPTS IN HYPERTENSIVE RETINOPATHY
Current Concepts In Hypertensive Retinopathy
The retinal physician is often the first to detect it.
MAYURI BHARGAVA, MD • TIEN Y. WONG, MD, PhD
|
Mayuri Bhargava, MD, and Tien Y. Wong, MD, PhD, serve on the faculty of the Singapore Eye Research Institute of the Singapore National Eye Centre, the Department of Ophthalmology at the Yong Loo Lin School of Medicine of the National University of Singapore, and the Eye Academic Clinical Program of Duke-NUS Graduate Medical School in Singapore. Neither author reports any financial interests in any products mentioned in this article. Dr. Wong can be reached via e-mail at tien_yin_wong@nuhs.edu.sg. |
The retinal vasculature undergoes a series of vascular changes associated with elevated blood pressure, widely known as hypertensive retinopathy. Since it was first described in the late 1800s in patients with hypertensive renal disease, researchers have suggested hypertensive retinopathy as a marker for target organ damage.
Physicians have used hypertensive retinopathy to predict risk of stroke, cardiovascular disease, and even mortality.1,2 As a result, the assessment of hypertensive retinopathy signs appears in clinical guidelines for the management of patients with hypertension.3,4
GROWTH IN KNOWLEDGE
Major advancements in research have enabled a better understanding of the epidemiology, pathogenesis, associated systemic conditions, and clinical implications of hypertensive retinopathy.3
Among the major advances are the use of digital fundus photography to capture hypertensive retinopathy signs, particularly early signs, and the application of computer-based techniques to measure early changes, such as generalized retinal arteriolar narrowing.3,5-7
Based on these research approaches and data, an updated classification of hypertensive retinopathy into mild, moderate and malignant stages appears to allow for easier clinical use.2,8
Newer retinal imaging tools can now provide precise, and even dynamic, quantification of subtle retinal vascular signs associated with hypertension. However, whether we can use these newer techniques clinically remains to be determined.
PATHOGENESIS AND PRESENTATION
Hypertensive retinopathy, first described as “albuminuric retinitis,”9 has traditionally been referred to as a spectrum of “retinal vascular signs” caused by elevated blood pressure.1
These signs include focal and generalized signs occurring consequent to initially physiological autoregulation of the vasculature. However, they subsequently relate to breakdown of the autoregulation pathways due to modulation in the dynamics of perfusion pressure and precapillary arterioles by endothelial-derived molecules (endothelins, thromboxane A2, prostaglandins, and nitric oxide).1,2
The focal or localized signs of hypertensive retinopathy consist of focal retinal arteriolar narrowing, arteriovenous nicking (Figure 1), and retinopathy-like lesions (microaneurysms, retinal hemorrhage, cotton wool spots) (Figure 2). At the same time, generalized or diffuse signs include generalized arteriolar narrowing (Figure 1) and arteriolar wall opacification (“silver” or “copper” wiring).2
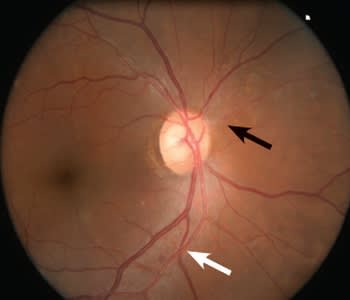
Figure 1. Mild hypertensive retinopathy: generalized and focal arteriolar narrowing (black arrow) with arteriovenous nicking (white arrow).
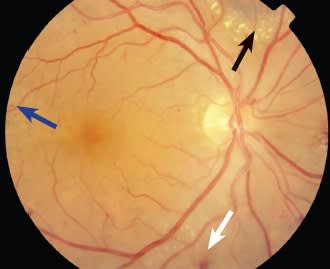
Figure 2. Moderate hypertensive retinopathy: arteriolar narrowing with arteriovenous nicking, flame-shaped hemorrhages (white arrow), dot blot hemorrhages (blue arrow), and hard exudates (black arrow).
Retinal arteriolar narrowing or attenuation is a defining sign of hypertensive retinopathy and is considered to be an autoregulatory physiological response to initial vasospasm of retinal arterioles. This sign appears even in children with hypertension.10
Long-term Consequences
If an individual’s blood pressure remains chronically elevated, the pathological process of arteriolosclerosis sets in, and the changes are generally irreversible. These permanent changes cause opacification of the arteriolar wall, sometimes known as “silver” or “copper” wiring, with consequent compression of venules due to atherosclerotic arteriolar changes, leading to arteriovenous nicking (AVN)11 (Figure 1).
Severe hypertension ultimately causes blood-retinal-barrier disruption, leading to an “exudative” stage, during which flame-shaped retinal hemorrhages and cotton wool spots appear.
Uncontrolled severe hypertension can lead to a “malignant” stage, with optic disc and macular edema due to raised intracranial pressure. This stage is associated with poor prognosis for survival.11
We present these pathophysiological changes and clinical features in detail in Table 1.
| Table 1. Pathophysiology of Hypertensive Retinopathy | ||
|---|---|---|
| Pathophysiological Mechanism | Signs | |
|
Acute hypertension |
|
|
|
Chronic hypertension |
|
|
|
Severe hypertension |
|
|
|
Accelerated hypertension |
|
|
CLASSIFICATION AND CLINICAL IMPLICATIONS
The traditional classification of hypertensive retinopathy, by Gunn12, 13 and Keith, Wagener, and Barker (KWB),14 categorizes the commonly observed hypertensive retinal signs into four grades of increasing severity (Table 2).
However, the usefulness of this classification in routine practice is questionable.15-19 Three major limitations exist. First, the classification has poor reliability and reproducibility among clinicians, particularly in distinguishing between the two lower grades of KWB retinopathy (grade 1 vs grade 2).20
Second, the KWB retinopathy grades do not correlate with severity of hypertension.21,22 Third, some researchers have interpreted the KWB grades as sequential in nature, but patients may have higher grades (grade 3) without going through lower grades of retinopathy (grade 1).
Finally, no good prospective studies have emerged to demonstrate that these four grades of retinopathy correlate with prognosis, cardiovascular events, and mortality. It is not clear, for example, that patients with KWB grade 2 retinopathy are at greater risk than patients with grade 1 retinopathy.
Recent Advances
Three major advances have occurred in research in hypertensive retinopathy over the past two decades. The first advance has been the broad application of retinal photography (initial film and subsequently digital) to capture hypertensive retinopathy signs in clinical studies. As a result, elements of reproducibility in measuring early signs are no longer necessary.
The second advance is the application of computer-based techniques to measure early changes, such as generalized retinal arteriolar narrowing. These techniques have allowed the third major advance: improved understanding of the relationship of these signs in large epidemiological studies with systemic conditions and target end organ damage, such as cerebrovascular, coronary, and renal diseases.
A Simplified Classification Scheme
As a result of this new understanding, we proposed a three-grade classification scheme (“simplified classification”),2 based on the strength of reported associations between hypertensive retinopathy and cardiovascular diseases, as well as target end organ damage (Table 2).
| Table 2. Keith–Wagener–Barker and ‘Simplified Classification’ Systems for Hypertensive Retinopathy | |||
|---|---|---|---|
| Keith–Wagener–Barker | Simplified Classification | ||
|
GRADE |
FEATURES |
GRADE |
FEATURES |
|
1 |
Mild generalized retinal arteriolar narrowing |
None |
No detectable signs |
|
2 |
Definite focal narrowing and arteriovenous nipping |
Mild |
Generalized arteriolar narrowing, focal arteriolar narrowing, arteriovenous nicking, opacity (“copper wiring”) of arteriolar wall, or a combination of these signs |
|
3 |
Signs of grade 2 retinopathy plus retinal hemorrhages, exudates, and cotton wool spots |
Moderate |
Retinal hemorrhages (blot-shaped, dot-shaped, or fiame-shaped), microaneurysms, cotton wool spots, hard exudates, or a combination of these signs |
|
4 |
Severe grade 3 retinopathy plus papilledema |
Malignant |
Signs of moderate retinopathy plus optic disk swelling |
In this simplified classification,2 mild retinopathy encompasses retinal arteriolar signs such as focal arteriolar narrowing and AVN. Moderate retinopathy includes retinopathy-like lesions, such as retinal hemorrhage, cotton wool spots, and hard exudates. The malignant stage comprises moderate retinopathy signs with optic disc or macular edema (Table 2).
Two recent studies have validated the clinical usefulness of this simplified classification for retinopathy detection. The first study reported good reliability and reproducibility of the simplified classification among optometrists and ophthalmologists.8 The second demonstrated that the classification predicted the long-term risk of stroke.23 As a result, the simplified classification appears to perform better than the KWB classification in terms of ease of use and appropriate stratification of cardiovascular risk disease.
EPIDEMIOLOGY
Over the past decade, substantial population-based studies have evaluated hypertensive retinopathy based on retinal photography (Table 3).4,10,24-33
Data from these population-based epidemiological studies indicate that hypertensive retinopathy signs frequently occur in 3% to 14% of nondiabetic adults age 40 years and older.
Among the different retinal signs, focal arteriolar narrowing and AVN occur in 7% to 12% of hypertensive people, respectively, and the most common retinopathy lesions observed are isolated retinal hemorrhages or microaneurysms (3%-17%), with cotton wool spots being relatively uncommon (0.3%). The 10-year cumulative incidence of these retinopathy signs is 16%.34
| Table 3. Prevalence of Hypertensive Retinopathy Signs* | |||
|---|---|---|---|
| STUDY | POPULATION | ALL PERSONS | HYPERTENSIVE PERSONS |
|
Beaver Dam Eye Study (BDES) |
4,926 people age 43 to 86 years, white ethnicity, Wisconsin |
7.8% to 13.5% |
2.8% to 19.4% |
|
Blue Mountains Eye Study (BMES) |
3,654 people age 49 and older, white ethnicity, Australia |
7.9% to 9.9% |
6.8% to 15.4% |
|
Atherosclerosis Risk In Communities (ARIC) Study |
10,000+ people age 51 to 72 years, white and black ethnicity, four US communities |
3.3% to 7.3% |
5% to 11.9% |
|
Atherosclerosis Risk In Communities (ARIC) Study — third examination |
2,907 hypertensives age 49 to 73 years, white and black ethnicity, four US communities |
51.6% |
|
|
Cardiovascular Health Study (CHS) |
2,400+ people age 69 to 97 years, white and black ethnicity, four US communities |
8.3% to 9.6% |
9% to 12.3% |
|
Rotterdam Eye Study |
5,674 people age 55 and older, white ethnicity, Netherlands |
5% (MAs + RH) |
-- |
|
Singapore Malay Eye Study (SiMES) |
3,280 Malays age 40 to 80 years, Asians, Singapore |
MAs + RH = 5.7%; CWS = 0.17%; FAN = 17.8%; AVN = 14.5% |
6% (MAs + RH = 5.8%; CWS = 0.3%) |
|
Handan Eye Study |
6,830 Chinese age >30 years, China |
8.6% |
12.1% |
|
Beijing Eye Study |
4,439 Chinese subjects, age 45-89 years, China |
GAN=14.6%; FAN=6.2%; AVN=6.1% |
7.7% (FAN, AVN, MAs/RH) |
|
Funagata Study |
1,961 Japanese age 35 or older, Japan |
12.5% (11.9 to 17.2%) = mostly MAs and RHs |
|
|
Multi-Ethnic Study of Ath-erosclerosis (MESA) |
6,176 subjects age 45-84 years, four ethnic groups (white, black, Hispanic, and Chinese) |
32% (MAs + RH + CWS) |
|
|
Chronic Renal Insufficiency Cohort (CRIC) |
1,936 white subjects age 21-74 years |
11% (MAs = 7.4%, RHs = 2.8%) |
MAs + RH = 6.29% |
|
Singapore Indian Eye Study (SINDI) |
3,100 Asian Indians, age 40-80 years, Singapore |
MAs + RH = 5.06% |
CWS = 0.3% |
|
Singapore Chinese Eye Study (SCES) |
2,700 Chinese, age 40-80 years, Singapore |
MAs + RH = 3.9% |
CWS = 0.14% |
| *Includes generalized arteriolar narrowing (GAN), focal arteriolar narrowing (FAN), arteriovenous nicking (AVN) and retinal hemorrhages (RH)/microaneurysms (MA) /cotton wool spots (CWS) in non-diabetic individuals
|
|||
Racial and Ethnic Variations
Racial and ethnic differences exist in the epidemiology of hypertensive retinopathy. Studies comparing racial and ethnic variations have shown that the highest rates of retinopathy appear in Chinese subjects (17.2%) and the lowest rates in white populations (11.9%) and populations of African descent (13.9%).
In Asian populations, Japanese31 and Malay people28 living in urban areas showed a lower incidence of retinopathy (7.7% and 6%, respectively) compared to rural Chinese (13.6%)29 populations.
The higher prevalence and lower awareness of hypertension, as well as poor blood pressure control among the rural population, may lead to a higher prevalence of retinopathy in rural areas.29,35
RELATIONSHIP WITH BLOOD PRESSURE
Several population- and clinic-based studies have demonstrated the relationship of hypertensive retinopathy signs with the severity of hypertension.10,24-33,36 The frequencies of generalized arteriolar narrowing (25.4% vs 14.6%), focal arteriolar narrowing (12.1% vs 6.2%), and AVN (12.3% vs 6.1%) are consistently higher in people with hypertension than in those without.30
According to the computer-assisted programs used in the Atherosclerosis Risk in Communities study, major systemic determinants of narrower arteriolar caliber included past and current higher blood pressure. However, wider venular caliber was only associated with current hypertension.37
In a recent follow-up report of the Beaver Dam Eye Study, narrower retinal arteriolar and venular caliber was independently associated with past and current blood pressure.38
Four Recent Observations
Several major observations have emerged from recent research. First, studies have shown in the hypertensive population, patients with elevated blood pressure despite medical therapy had a higher frequency of retinopathy signs, compared with those whose blood pressure was controlled or those who were normotensive.32 This finding indicates that hypertensive retinopathy signs may be an indicator of blood pressure control.
Second, the patterns of specific retinal vascular changes vary with current and past blood pressure levels. Generalized retinal arteriolar narrowing and AVN usually appear in patients with long-term hypertension and are independently associated with past blood pressure levels measured up to 10 years before retinal assessment.39
In contrast, focal arteriolar narrowing and retinopathy lesions (retinal hemorrhages, microaneurysms, and cotton wool spots) may indicate more transitory blood pressure changes and are related only to concurrently measured blood pressure.40
Third, the association between blood pressure and retinal microvascular signs is weaker with age, possibly reflecting greater sclerosis of retinal arterioles in older persons.5
Fourth, longitudinal data from recent population-based studies have demonstrated that smaller retinal arteriolar and larger venular calibers precede clinical stages of hypertension5 and predict the risk of hypertension in initially normotensive individuals.41,42
RELATIONSHIP WITH OTHER RISK MARKERS
In addition to blood pressure, researchers have also associated hypertensive retinopathy signs with other vascular risk markers, such as inflammation (Creactive protein, fibrinogen),33,43,44 endothelial cell activation (von-Willebrand factor),43 angiogenesis,45 adiponectin,46 and leptin.47
Recent data have also suggested a positive correlation between serum ferritin levels and the degree of hypertensive retinopathy, presumably due to increased levels of oxidative stress.48 Lower birth weight,49 smoking status, body mass index, and heavy alcohol consumption are also associated with narrower retinal arteriolar diameters, which are the earliest changes in hypertensive retinopathy.38
Genetic Factors
Some evidence also exists for a genetic influence on hypertensive retinopathy changes. A twin study of retinal vascular caliber reported that the heritabilities of retinal arteriolar and venular calibers were 70% and 83%, respectively.50
Genome-wide linkage studies found that the regions for retinal vascular caliber overlapped with those that were previously associated with essential hypertension, coronary heart disease, and vasculogenesis.5
Polymorphisms of apolipoprotein E and angiotensin-converting enzyme I/D genes,51 chromosome 6p21.1,52 novel chromosome 5,53 and novel locus 12q24,54 have shown weak linkages with retinal vascular caliber.
The significance of these risk factors’ associations with hypertensive retinopathy, however, remains unclear,25 warranting further investigation.
NEW RETINAL IMAGING TECHNIQUES
Novel computer-assisted techniques of retinal imaging allow for quantification of subtle hypertensive retinopathy changes, enhancing our understanding of retinal microcirculatory alterations.
For example, computer-based methods for measuring retinal vascular caliber (Figure 3) have allowed studies to demonstrate that retinal arteriolar caliber is strongly associated with current, past, and future levels of blood pressure, in both adults and children.6

Figure 3. Retinal vessel caliber software: Singapore I Vessel Assessment (SIVA) used for measuring retinal arteriolar and venular diameters.
Also, researchers have linked retinal venular caliber with concurrent elevated blood pressure, suggesting that venules may represent a dynamic component in response to hypertension.7,55
Additional software now exists for objective assessment of microvascular remodeling, using novel retinal vascular features, such as branching angles, bifurcation, fractal dimension, tortuosity, vascular length-to-diameter ratio, and wall-to-lumen ratio.6
Newer Evidence and Technology
In a recent study in multiethnic Asian subjects, the authors associated hypertension with a sparser retinal vascular network, using retinal vascular fractal dimensions in subjects with uncontrolled or untreated hypertension.56
In addition, studies have demonstrated that increased retinal venular tortuosity, smaller fractal dimension, smaller arteriolar branching asymmetry ratio, and increased wall-to-lumen ratio are associated with uncontrolled and untreated hypertension.37
Normotensive people also demonstrate these retinal vascular changes in various ethnic groups, so they may prove useful in predicting future risk of hypertension and cardiovascular diseases.57
Further technological advancements in retinal imaging have allowed for the evaluation of dynamic retinal vascular flow and remodeling, via tools such as the dynamic vessel analyzer and scanning laser Doppler flowmetry.
Innovative retinal imaging tools, such as ultrawide-field retinal imaging, adaptive optics, retinal oximetry, and Doppler optical coherence tomography, have emerged recently to evaluate in advance the link between the eye and hypertension.6
RELATIONSHIP WITH TARGET ORGAN DAMAGE
The concept that signs of hypertensive retinopathy are markers of systemic vascular damage elsewhere, such as the brain and kidney, is more than a decade old.16 Since then, numerous studies have reported a strong link between hypertensive retinopathy and the risk of incident and future cerebrovascular events.
In the Atherosclerosis Risk in Communities (ARIC) study, subjects with hypertensive retinopathy were not only at an increased risk of developing incident stroke,58,59 but also cognitive decline,60 cerebral white matter lesions,59 and cerebral atrophy,61 even after controlling for traditional risk factors.
Additionally, studies have associated generalized arteriolar narrowing, retinal venular widening,62 focal arteriolar narrowing, and AVN with lacunar stroke,62,63 while others have linked retinopathy signs to nonlacunar thrombotic, cardioembolic stroke61 and intracerebral hemorrhage.64,65
A recent report on a follow-up to the ARIC study showed that hypertensive retinopathy predicted the long-term risk of stroke.23 In this study of hypertensive patients with no previous cerebrovascular disease, 5.6% developed stroke, after a mean follow-up period of 13 years.
Incident stroke (19.3% vs 4.3%) and incident cerebral infarction (15.5% vs 3.6%) were significantly more common in people with retinopathy than those without retinopathy.
Even among subjects with hypertension receiving treatment with adequate control of blood pressure, those with mild and moderate retinopathy had cerebral infarction.
Kidney and Heart Disease
The link between hypertensive retinopathy and renal impairment, demonstrated in various cross-sectional studies,66-68 has had more conflicting results.
Retinal arteriolar narrowing was associated with the risk of chronic kidney disease (CKD) and microalbuminuria,69 after adjusting for traditional risk factors in different studies, including the Atherosclerosis Risk in Communities study.70,71
However, the Cardiovascular Health Study found no association between hypertensive retinopathy and CKD among elderly adults.72 In another study, retinal venular caliber was also associated with CKD.73
Hypertensive retinopathy is also associated with heart disease. Retinal arteriolar narrowing is closely linked to decreased myocardial blood flow and perfusion reserve.74
Patients with moderate hypertensive retinopathy have more than twice the risk of developing congestive heart failure compared to those without retinopathy.75 Other studies have strongly associated retinopathy with coronary artery disease in elderly hypertensives76 and those with markers of subclinical or microvascular coronary disease,77 especially in women with type 1 diabetes.78
Focal arteriolar narrowing, AVN, and moderate hypertensive retinopathy are also related to left ventricular hypertrophy and carotid thickening and plaques among hypertensives.79,80
Newly Identified Risks
Emerging evidence has suggested that the risk for coronary heart disease is greater in women previously considered “low risk” by traditional risk factors.81,82
The Multi-Ethnic Study of Atherosclerosis associated increased internal carotid intima media thickness with retinopathy in nondiabetics, especially white and Latino subjects.25
Another study evaluating retinopathy and cardiovascular disease in kidney disease patients showed strong associations between hypertensive retinopathy signs and cardiovascular disease and stroke, after adjusting for traditional risk factors.83
Other data have indicated that hypertensive subjects exhibit progressive stiffening of the aorta, in parallel with the progression of retinal alterations.84
Finally, many large studies have shown a strong correlation between hypertensive retinopathy and cardiovascular mortality.85,86 For example, in a large Japanese study,87 both hypertensive and nonhypertensive subjects with mild retinopathy signs were at higher risk for cardiovascular mortality, independent of traditional cardiovascular risk factors.
CLINICAL MANAGEMENT
The management of patients with hypertensive retinopathy should be based on the “simplified classification”2 shown in Table 4. This scheme allows clinicians to utilize retinal microvasculature as a model for assessing hypertensive patients for target organ damage risk stratification.
Patients with mild retinopathy signs will likely only require routine care of blood pressure, according to established guidelines. Patients with moderate retinopathy signs may benefit from further assessment of vascular risk (eg, assessment of kidney damage or left ventricular hypertrophy).
| Table 4. Risk Stratification and Management Guidelines of Hypertensive Retinopathy | |||
|---|---|---|---|
| Retinopathy Grade | Description | Systemic Associations | Management |
|
Mild |
One or more of the following signs: Generalized arteriolar narrowing, focal arteriolar narrowing, arteriovenous nicking, arteriolar wall opacity (silver-wiring) |
Weak associations with stroke, coronary heart disease and cardiovascular mortality |
|
|
Moderate |
Mild retinopathy with one or more of the following signs: Retinal hemorrhage (blot, dot or flame-shaped), microaneurysms, cotton wool spot, hard exudates |
Strong association with stroke, congestive heart failure, renal dysfunction, and cardiovascular mortality |
|
|
Malignant |
Moderate retinopathy signs plus optic disc swelling and macular edema |
Associated with mortality |
|
If clinically indicated, these patients can receive appropriate risk reduction therapy. Patients with malignant retinopathy will need urgent antihypertensive management.2
Antihypertensive medication may reverse hypertensive retinopathy signs, with clinical case series88,89 showing regression of some retinopathy signs (eg, hemorrhages, cotton wool spots) with control of blood pressure.
Newer studies, based on digital retinal photography and computerized analysis, have revealed that blood pressure reduction is associated with a reduction in arteriolar narrowing, widening of arteriolar branch angle, and an increase in arteriolar density among untreated hypertensives.90
Medical Therapies and Retinal Assessment
It is unclear whether differential effects of antihypertensive therapy exist on retinal signs. Both calcium channel blockers and angiotensin antagonists cause regression of retinal vascular signs to similar extents.90
Nevertheless, in one study comparing them to beta-blockers, calcium antagonists showed a better remodeling response in retinal microvasculature, possibly due to the vasodilatory action of the drug.91
A retinal assessment may be useful to screen for patients with “white coat” or “masked hypertension,” common in 12% to 30% of elderly women.92 This subset represents an intermediate category between normotensives and “real” or sustained hypertensives with regard to target organ damage and cardiovascular risk. Detection of hypertensive retinopathy in subjects with white coat hypertension may indicate the need for antihypertensive therapy.93
In patients with malignant hypertension with optic disc and macular edema, in addition to systemic management of blood pressure, ocular adjuvant treatments that target VEGF reduction have come to the forefront.
These anti-VEGF agents presumably act by reducing vascular permeability, and they reduce macular edema in eyes with malignant hypertension. Although a few anecdotal reports94-96 available in the literature have shown good visual outcomes, the routine use of such therapies warrants further research.
CONCLUSION
Evaluation of hypertensive retinopathy signs, using digital retinal photography, in hypertensive patients should continue to be part of the guidelines for management of hypertension. We should encourage the use of a “simplified classification” for hypertensive retinopathy in clinical practice because we can easily apply clinically observable signs to stratify patients at risk of cardiovascular disease.
Novel retinal vascular imaging has the potential for noninvasive assessment of the microvascular sequelae of hypertension. While currently confined to research settings, this modality may play a clinical role in the future. RP
REFFERENCES
1. Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132-1145.
2. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310-2317.
3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281-1357.
4. Taylor J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2108-2109.
5. Sun C, Wang JJ, Mackey DA, et al. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74-95.
6. Cheung CY, Ikram MK, Sabanayagam C, et al. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094-1103.
7. Cheung CY, Tay WT, Mitchell P, et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29:1380-1391.
8. Downie LE, Hodgson LAB, D’Sylva C, et al. Hypertensive retinopathy: comparing the Keith-Wagener-Barker to a simplified classification. J Hypertens. 2013;31:960-965.
9. Liebreich R. [Ophthalmoscopic findings in Bright’s disease]. Graefes Arch Clin Exp Ophthalmol. 1859;5:265-268.
10. Gopinath B, Baur LA, Wang JJ, et al. Blood pressure is associated with retinal vessel signs in preadolescent children. J Hypertens. 2010 Jul;28:1406-1412.
11. Wong TY, Mitchell P The eye in hypertension. Lancet. 2007;369:425-435.
12. Gunn RM. Ophthalmoscopic evidence of (1) arterial changes associated with chronic renal diseases and (2) of increased arterial tension. Trans Am Ophthalmol Soc. 1892;12:124-125.
13. Gunn RM. On ophthalmoscopic evidence of general arterial disease. Trans Ophthalmol Soc U K. 1898;18:356-381.
14. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1939;197:332-343.
15. Dodson PM, Lip GY, Eames SM, et al. Hypertensive retinopathy: a review of existing classification systems and a suggestion for a simplified grading system. J Hum Hypertens. 1996;10:93-98.
16. Wong TY, Klein R, Klein BE, et al. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59-80.
17. Chatterjee S, Chattopadhyay S, Hope-Ross M, et al. Hypertension and the eye: changing perspectives. J Hum Hypertens. 2002;16:667-675.
18. Cuspidi C, Salerno M, Salerno DE, et al. High prevalence of retinal vascular changes in never-treated essential hypertensives: an inter- and intra-observer reproducibility study with nonmydriatic retinography. Blood Press. 2004;13:25-30.
19. Cuspidi C, Negri F, Giudici V, et al. Retinal changes and cardiac remodelling in systemic hypertension. Ther Adv Cardiovasc Dis 2009;3:205-214.
20. Dimmitt SB, West JN, Eames SM, et al. Usefulness of ophthalmoscopy in mild to moderate hypertension. Lancet. 1989;1:1103-1106.
21. Fuchs FD, Maestri MK, Bredemeier M, et al. Study of the usefulness of optic fundii examination of patients with hypertension in a clinical setting. . J Hum Hypertens. 1995;9:547-551.
22. Cuspidi C, Meani S, Salerno N, et al. Retinal microvascular changes and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:2095-2102.
23. Ong Y-T, Wong TY, Klein R, et al. Hypertensive Retinopathy and Risk of Stroke. Hypertension 2013;62:706-711
24. Wang JJ, Baker ML, Hand PJ, et al. Transient ischemic attack and acute ischemic stroke: associations with retinal microvascular signs. Stroke. 2011;42:404-408.
25. Ojaimi E, Nguyen TT, Klein R, et al. Retinopathy signs in people without diabetes. The multi-ethnic study of atherosclerosis. Ophthalmology. 2011;118:656-662.
26. Huang OS, Lamoureux E, Tay WT, et al. Glycemic and blood pressure control in an Asian Malay population with diabetes and diabetic retinopathy. Arch Ophthalmol. 2010;128:1185-1190.
27. Kawasaki R, Cheung N, Mosley T, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:1826-1828.
28. Jeganathan V, Cheung N, Tay W, et al. Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay Eye Study. Arch Ophthalmol. 2010;128:40-45.
29. Peng XY, Wang FH, Liang YB, et al. Retinopathy in persons without diabetes: the Handan Eye Study. Ophthalmology. 2010;117:531-537.
30. Wang S, Xu L, Jonas JB, et al. Major eye diseases and risk factors associated with systemic hypertension in an adult Chinese population: the Beijing Eye Study. Ophthalmology. 2009;116:2373-2380.
31. Kawasaki R, Wang JJ, Wong TY, et al. Impaired glucose tolerance, but not impaired fasting glucose, is associated with retinopathy in Japanese population: the Funagata study. Diabetes Obes Metab. 2008;10:514-515.
32. Wang JJ, Mitchell P Leung H, et al. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension 2003;42:534-541.
33. Wong TY, Klein R, Sharret AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older people. The Cardiovascular Health Study. Ophthalmology. 2003;110:658-666.
34. Wang JJ, Rochtchina E, Kaushik S, et al. Long-term incidence of isolated retinopathy lesions in older persons without diabetes: the Blue Mountains Eye Study. Invest Opthalmol Vis Sci. 2010;51:ARVO E-Abstract 1236.
35. Yip W, Wong TY, Jonas JB, et al. Prevalence, awareness, and control of hypertension among Asian Indians living in urban Singapore and rural India. J Hypertens. 2013 8:1539-1546.
36. Kawasaki R, Tielsch JM, Wang JJ, et al. The metabolic syndrome and retinal microvascular signs in a Japanese population: the Funagata study. Br J Ophthalmol. 2008;92:161-166.
37. Liew G, Sharett AR, Wang JJ, et al. Relative importance of systemic determinants of retinal aeteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126:1404-1410.
38. Klein R, Myers CE, Knudtson MD, et al. Relationship of blood pressure and other factors to serial retinal arteriolar diameter measurements over time: the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1019-1027.
39. Leung H, Wang JJ, Rochtchina E, et al. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens. 2004;22:1543-1549.
40. Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44:2900-2904.
41. Tanabe Y, Kawasaki R, Wang JJ, et al. Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: the Funagata study. Microcirculation. 2010;17:94-102.
42. Smith W, Wang JJ, Wong TY, et al. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44:442-447.
43. Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644-1650.
44. Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129-2134.
45. Tsai WC, Li YH, Huang YY, et al. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci (Lond). 2005;109:39-43.
46. Yilmaz MI, Sonmez A, Kilic S, et al. The association of plasma adiponectin levels with hypertensive retinopathy. Eur J Endocrinol. 2005;152:233-240.
47. Uckaya G, Ozata M, Sonmez A, et al. Is leptin associated with hypertensive retinopathy? J Clin Endocrinol Metab. 2000;85:683-687.
48. Coban E, Alkan E, Altuntas S, et al. Serum ferritin levels correlate with hypertensive retinopathy. Med Sci Monit. 2010;16:92-95.
49. Sasongko MB, Wong TY, Wang JJ. Retinal arteriolar changes: intermediate pathways linking early life exposures to cardiovascular disease? Microcirculation. 2010;17:21-31.
50. Taarnhoj NC, Larsen M, Sander B, et al. Heritability of retinal vessel diameters and blood pressure: a twin study. Invest Ophthalmol Vis Sci. 2006;47:3539-3544.
51. Tanabe Y, Kawasaki R, Wang JJ, et al. Angiotensin-converting enzyme gene and retinal arteriolar narrowing: the Funagata Study. J Hum Hypertens. 2009;23:788-793.
52. Cheng CY, Reich D, Wong TY, et al. Admixture mapping scans identify a locus affecting retinal vascular caliber in hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) study. PLoS Genet. 2010;6:e1000908.
53. Sim X, Jensen RA, Ikram MK, et al. Genetic loci for retinal arteriolar microcirculation. PLoS One. 2013;8:e65804.
54. Ikram M, Xueling S, Jensen R, et al. Four novel loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS Genet. 2010;6:e1001184.
55. Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007-1013.
56. Sng CC, Wong WL, Cheung CY, et al. Retinal vascular fractal and blood pressure in a multiethnic population. J Hypertens. 2013 Jun 19. [Epub ahead of print]
57. Li X, Wong WL, Cheung CY, et al. Racial differences in retinal vessel geometric characteristics: a multiethnic study in healthy Asians. Invest Ophthalmol Vis Sci. 2013;54:3650-3656.
58. Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134-1140.
59. Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67-74.
60. Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke. 2002;33:1487-1492.
61. Wong TY, Mosley TH, Jr. , Klein R, et al. Retinal microvascular changes and MRI signs of cerebral atrophy in healthy, middle-aged people. Neurology. 2003;61:806-811.
62. Yatsuya H, Folsom AR, Wong TY, et al. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349-1355.
63. Lindley RI, Wang JJ, Wong MC, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 2009;8:628-634.
64. Wieberdink RG, Ikram MK, Koudstaal PJ, et al. Retinal vascular calibers and the risk of intracerebral hemorrhage and cerebral infarction: the Rotterdam Study. Stroke. 2010;41:2757-2761.
65. Baker ML, Hand PJ, Wong TY, et al. Retinopathy and lobar intracerebral hemorrhage: insights into pathogenesis. Arch Neurol. 2010;67:1224-1230.
66. Saitoh M, Matsuo K, Nomoto S, et al. Relationship between left ventricular hypertrophy and renal and retinal damage in untreated patients with essential hypertension. Intern Med. 1998;37:576-580.
67. Sabanayagam C, Tai ES, Shankar A, et al. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J Hypertens. 2009;27:2209-2217.
68. Sabanayagam C, Shankar A, Koh D, et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol. 2009;169:625-632.
69. Lim LS, Cheung CY, Sabanayagam C, et al. Structural changes in the retinal micro-vasculature and renal function. Invest Ophthalmol Vis Sci. 2013;54:2970-2976.
70. Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15:2469-2476.
71. Yau JW, Xie J, Kawasaki R, et al. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2011;58:39-46.
72. Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46:214-224.
73. Myers CE, Klein R, Knudtson MD, et al. Determinants of retinal venular diameter: the Beaver Dam Eye Study. Ophthalmology. 2012;119:2563-2571.
74. Wang L, Wong TY, Sharrett AR, et al. Relationship between retinal arteriolar narrowing and myocardial perfusion: Multi-Ethnic Study of Atherosclerosis. Hypertension. 2008;51:119-126.
75. Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63-69.
76. Shantha GP, Srinivasan Y, Kumar AA, et al. Can retinal changes predict coronary artery disease in elderly hypertensive patients presenting with angina? Am J Emer Med. 2010;28:617-621.
77. Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling. J Am Coll Cardiol. 2007;50:48-55.
78. Miller RG, Prince CT, Klein R, et al. Retinal vessel diameter and the incidence of coronary artery disease in type 1 diabetes. Am J Ophthalmol. 2009;147:653-660.
79. Kim G-H, Youn H-J, Kang S, et al. Relation between grade II hypertensive retinopathy and coronary artery disease in treated essential hypertensives. Clin Exp Hypertens. 2010;32:469-473.
80. Cuspidi C, Meani S, Valerio C, et al. Prevalence and correlates of advanced retinopathy in a large selected hypertensive population. The Evaluation of Target Organ Damage in Hypertension (ETODH) study. Blood Press. 2005;14:25-30.
81. McClintic BR, McClintic JI, Bisognano JD, et al. The relationship between retinal microvascular abnormalities and coronary heart disease: a review. Am J Med. 2010;123:e1-e374.e7.
82. Shaw LJ, Lewis JF Hlatky MA, et al. Women’s Ischemic Syndrome Evaluation: current status and future research directions, report of the National Heart, Lung, and Blood Institute Workshop October 2-4, 2002, Section 5: gender-related risk factors for ischemic heart disease. Circulation. 2004;109:e56-e58.
83. Grunwald J, E. , Ying G-S, Maguire M, et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort (CRIC study). Am J Cardiol 2012;110:246-253.
84. Katsi V, Vlachopoulos C, Souretis G, et al. Association between retinal microcirculation and aortic stiffness in hypertensive patients. Int J Cardiol. 2012;157:370-373.
85. Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933-940.
86. Liew G, Wong TY, Mitchell P, et al. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95:391-394.
87. Sairenchi T, Iso H, Yamagishi K, et al. Mild retinopathy is a risk factor for cardiovascular mortality in Japanese with and without hypertension: the Ibaraki Prefectural Health Study. Circulation. 2011;124:2502-2511.
88. Bock KD. Regression of retinal vascular changes by antihypertensive therapy. Hypertension. 1984;6(Pt 2):158-162.
89. Dahlof B, Stenkula S, Hansson L. Hypertensive retinal vascular changes: relationship to left ventricular hypertrophy and arteriolar changes before and after treatment. Blood Press. 1992;1:35-44.
90. Hughes AD, Stanton AV, Jabbar AS, et al. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26:1703-1707.
91. Thom S, Stettler C, Stanton A, et al. Differential effects of antihypertensive treatment on the retinal microcirculation. An Anglo-Scandinavian Cardiac Outcomes Trial substudy. Hypertension. 2009;54:405-408.
92. Karter Y, Curgunlu A, Altinisik S, et al. Target organ damage and changes in arterial compliance in white coat hypertension. Is white coat innocent? Blood Press. 2003;12:307-313.
93. Grosso A, Veglio F Porta M, et al. Hypertensive retinopathy revisited: some answers, more questions. Br J Ophthalmol. 2005;89:1646-1654.
94. Zucchiatti I, Iacono P, Battaglia PM, et al. Intravitreal bevacizumab in a patient with a macular star in malignant hypertension. Eur J Ophthalmol. 2011;21:336-339.
95. Kim EY, Lew HM, Song JH. Effect of intravitreal bevacizumab (Avastin(®)) therapy in malignant hypertensive retinopathy: a report of two cases. J Ocul Pharmacol Ther. 2012;28:318-322.
96. Salman AG. Intravitreal bevacizumab in persistent retinopathy secondary to malignant hypertension. Saudi J Ophthalmol. 2013;27:25-29.








