The Use of Macular Microperimetry in the Assessment and Diagnosis of Macular Disease
Microperimetry in retina is advancing.
David Squirrell, Franzco • Rita Ehrlich, MD
Data derived from automated macular microperimetry is increasingly being reported in the study of a variety of retinal and optic nerve disorders. A prerequisite for an understanding of what these data represent is an underlying knowledge of the capabilities and limitations of these instruments.
In this article, we will briefly review the use of the microperimeter in the diagnosis and assessment of macular disease. This discussion will focus on the more widely used Nidek MP1 microperimetry (Nidek Technologies, Padova, Italy). Other instruments with slightly differing specifications (Optuk Instrumentation OCT/SLO [OSLO]) do exist, but the discussion will focus on the Nidek MP1, as all microperimeters are based on broadly similar technological principles.
THE ROLE OF MICROPERIMETRY
Although BCVA remains the gold standard assessment tool for measuring visual function, it is widely recognized that conventional tests of vision, such as high-contrast BCVA, underestimate the actual level of visual impairment, particularly in older patients.1-6
As a result, other clinical assessment tools, such as contrast sensitivity, macular recovery function, and reading speed tests, are often employed to assess visual function in an individual, particularly when measuring changes in visual function after an intervention.7
Although the Humphrey Field Analyzer (HFA) can be used to assess central macular sensitivity, its role in monitoring macular disease has been limited by its inability to quantify retinal thresholds accurately over small and discrete retinal lesions and to retest these areas accurately over time.
In response to these limitations, the scanning laser ophthalmoscope (SLO) microperimeter was developed. The SLO integrates fundus imaging with computerized threshold perimetry to achieve an exact correlation between macular and corresponding functional defects.
This device was, however, very time-consuming and cumbersome to use and did not easily facilitate automated follow-up examinations.8 Consequently, its use remained the preserve of a few academic institutions, and it never gained widespread popularity as an assessment tool.
The desire for a more practical, user-friendly alternative led to the development of the Nidek MP1 microperimeter. This later iteration of microperimeters incorporates a color fundus camera for image registration and an auto-tracking system to facilitate the accurate measurement of retinal sensitivity within the central visual field, even in patients with unstable or extrafoveal fixation.
The MP1 also allows for automated follow-up examinations at the same retinal loci using its image-registration facility. Although the MP-1 has an array of test strategies, in clinical practice, it has two principle modes of use: fixation localization and threshold testing. We shall briefly describe the psychophysics of these two modes and review their clinical use in assessing and diagnosing macular disease.
THE ASSESSMENT OF FIXATION PATTERNS
The study of fixation patterns is beginning to receive attention from investigators, who are using them to measure visual performance and to understand and develop strategies for rehabilitating patients with macular disease. During testing, autotracking within the MP1 detects and measures patients' eye movements, calculating the shifts relative to central fixation at a frequency of 25 Hz (40 ms).
Consequently, the MP1 allows for fast, reliable microperimetric examination of fixation and scotoma characteristics in patients with macula disease, even when the visual acuity is reduced and fixation is eccentric and unstable (Figure 1).

Figure 1. Fixation plots obtained from a patient with wet AMD undergoing treatment with intravitreal ranibizumab. At the onset of treatment, the patient's fixation can clearly be seen to be eccentric and unstable. Six months after successful treatment, the patient's visual acuity has improved, and the fixation can be demonstrated to have become foveal and stable.
The widely accepted description of the stability and location of fixation is based upon Fujii et al.'s original classification,9 the parameters of which are now incorporated within the MP1 test report. In brief, the location of fixation is defined as the position of fixation with respect to the center of the foveal avascular zone, and stability of fixation is defined as the ability of the eye to maintain a stable fixation in the preferred retinal locus (PRL).
There are a limited, but growing, number of studies investigating fixation stability and location. Before the advent of the MP1, it was appreciated that good vision was a prerequisite for stable and steady fixation. By analyzing the relationship among MP1, OCT, and autofluorescence data, we now know that, in the context of AMD at least, stable and central fixation correlates well with preservation of the outer retinal signal on OCT.
Conversely, the presence of fibrosis, RPE atrophy, and loss of foveal autofluorescence are associated with a much higher rate of unstable eccentric fixation.10 Perhaps not surprisingly then, fixation is significantly more likely to be central and stable in patients with neovascular AMD who have received treatment with ranibizumab, compared to those who have not.11
Conversely, it has also been reported that patients can maintain a reasonable visual acuity with established eccentric fixation. This relative preservation of vision appears to be related to the stability of the eccentric fixation,12,13 and visual rehabilitation strategies are therefore being developed to exploit this phenomenon.
Existing published guidelines for the treatment of neovascular AMD14 have visual acuity and sub-/intraretinal fluid and hemorrhage as parameters to continue or stop treatment. However, in patients with a visual acuity of less than 20/80, only 17% were found to have foveal fixation. Furthermore, when the visual acuity was 20/200, this figure dropped to just 5%.13
These data suggests that below a visual acuity of 20/80, the decision to treat should not just be based on these traditional parameters alone, but it should also include an assessment as to whether disease reactivation threatens the new PRL.
THRESHOLD MICROPERIMETRY IN MACULAR DISEASE
Conventional static perimetry has long been the “gold standard” in the assessment of retinal sensitivity in the management of glaucoma. Macular microperimetry, like conventional perimetry, is a psychophysiological method that assesses retinal sensitivity. However, unlike convention perimetry, the automated eye-tracking system shifts the position of the stimulus to compensate for small eye movements, while larger eye movements prompt the test to pause until the patient refixates on the target. This auto-tracking and automatic registration of one test to another allow for precise microperimetric assessment of central field sensitivities.
Conceptually, the perimetric assessment from field analyzers, such as the HFA and MP1, are similar, but important differences in stimulus configuration can produce discordant results. Whereas the HFA perimeter uses a projection system with a broad range of stimulus intensities, the MP1 uses a small solid-state monitor to present the targets over a limited range of intensities.
Stimuli are presented on a background of 10 candelas per meter square (cd/m2) with the HFA, but on a dimmer background of 1.27 cd/m2 with the MP1. The differences in background adaptation level and minimum stimulus luminance cause a variation between the dynamic ranges of the two devices.
In psychophysics, thresholds are standardly measured and expressed as increments above the background level. Thus, the dB values for the HFA and MP1 represent attenuation from different maximum values, and to compare the results obtained from the MP1 and HFA, one must convert the data into equivalent threshold values.
Comparisons between the MP1 and HFA reveal some important findings relevant to our understanding of the results the MP1 obtains.15,16 The current range of the MP1 is 0-20 dB (0 dB represents the brightest luminance of 127 cd/m2). However, because the background luminance with the MP1 is dimmer, its dynamic range when converted to HFA equivalents is narrower, at 14-34 dB.
Furthermore, the first contrast step displayed by the MP1 is also considerably larger than the minimum threshold increment needed for a normally sighted subject to see the target, which leads to a ceiling effect at the top of its retinal-sensitivity threshold.
Clinical practice data support this limitation, as patients with healthy macula often record thresholds of 20 dB across the entire macular field, with no discernable difference in sensitivity between the fovea and parafoveal macula. With a lower range of 14dB (HFA equivalent), there is also a potential floor effect at higher stimuli. The limited dynamic range of the MP1 and, in particular, its lack of ability to discern small changes from “normal” are potential limitations of the current instruments.
Aside from this limitation, the MP1 affords the operator a great deal of flexibility in determining how the target area will be tested. In brief, the operator chooses from a menu of test patterns that can be projected across the target area. The operator can change the number, as well as the density, of test loci within the test polygon, which may be centered on any area of the central macula the operator chooses.
The operator can also choose which gating strategy to employ during the examination. A 4-2-1 double-gated strategy will be more accurate than a 4-2 gated strategy, but it takes longer and thus runs the risk of patient fatigue. Consequently, the designated test strategy will always be a compromise between test accuracy and time required. As yet, there has been no attempt to standardize the collection and reporting of MP1 data, and this makes comparison of reported data problematic.
One advantage of the MP1 is its potential to monitor macular function over time (Figure 2). To take advantage of this function, it is vital to know first what represents a “real” difference from one test to another, and this difference can be evaluated mathematically by deriving the 95% repeatability coefficient.
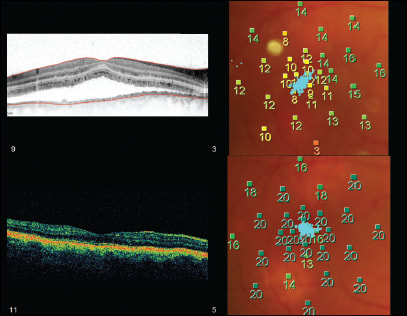
Figure 2. OCT and retinal threshold sensitivity maps of a patient with CSC before and after receiving PDT. The color-graded figures are the sensitivity (dB) recorded at each of the test loci. Superimposed on the MP map is the fixation plot. Although prior to treatment the patient had a high-contrast Snellen visual acuity of 20/30, the MP map clearly shows a large central scotoma and unstable fixation, both of which improved markedly after treatment.
This value represents the range that, under similar test conditions, one would expect the intertest variation of 95% of patients to lie within. In other words, any greater change in the mean retinal sensitivity from one test to another could be regarded as a statistically significant from baseline.17
Conversely, any change less than this value may simply be the inherent variation one would expect to occur between two different tests according to chance. The repeatability coefficient one derives for the MP1 is complicated by the result depending upon the gating strategy and test pattern employed. To date, few authors have reported the repeatability coefficient of their test strategies, and in all cases, different test strategies were used.
Nevertheless the reported repeatability coefficients obtained from these studies were broadly similar, with values of between 1.5 and 2.04 dB when the central visual field was tested.18-20
While one must be mindful of the limitations of the current instruments, threshold microperimetry is increasingly used in the assessment of a wide range of retinal pathologies. At a basic level, these data reveal that reliance on the commonly used “gold standard,” high-contrast Snellen visual acuity (by not measuring scotoma size, density, and fixation), not only risks understating the impact and morbidity of macular disease but also the beneficial effect of treatment on these as yet often-ignored parameters.18,21,22
However, perhaps the most exciting potential with microperimetry is that it allows investigators to assess the relationship between functional and structural changes accurately (Figures 3 and 4). For example, recent data have revealed that patients with established geographic atrophy have a progressive loss of macular function over time, which involves not only expansion of the absolute scotoma but also reduced sensitivity in the perilesional areas and a loss of fixation stability.23

Figure 3. The functional microperimetric map overlaid on the fluorescein angiogram of a patient with ischemic diabetic maculopathy. The patient's visual acuity was a remarkable 20/30, suggesting that the foveal and parafoveal tissue was being maintained in a larger part by the choriocapillaris.

Figure 4. Functional microperimetric and fixation maps overlaid on the fluorescein angiogram of a patient with an occult choroidal neovascular membrane. The patient recorded a Snellen visual acuity of 20/20. The functional MP map clearly shows the patient's depressed central retinal sensitivity, which was the cause of the patient's symptoms.
This loss of perilesional retinal sensitivity appears to be unrelated to progressive atrophy but is associated with progressive decreased fundus autofluorescence. However, the correlation between microperimetric changes and FAF changes remains weak.23,24
The use of MP data in patients with dry AMD promises not only to lead to a better understanding of the mechanisms involved in the natural history of progression of visual loss in dry AMD, but it should also lead to the development of protocols that will allow us to monitor more precisely the efficacy of interventions designed to treat acquired and congenital macular disease.25,26
Similar studies using microperimetry have also been conducted in patients with CSCR, to assess treatment21, 27-30 and also to understand better the pathophysiology of visual loss associated with this condition.31
CONCLUSION
In conclusion, the arrival of affordable and user-friendly automated microperimeters promises to enhance our understanding of macular disease and our assessment of future and existing treatments. The principal limitation of the existing microperimeters is their narrow dynamic range, although this may be overcome in later instruments, as users demand greater sensitivity at the top and bottom end of the existing ranges.
One must also ensure that any change reported is greater than the 95% repeatability coefficient and is thus a “real” change. To date, very few studies have produced these data, and those that have done so have used differing test strategies, making comparison problematic.
Looking forward, perhaps the most pressing need at this time is to standardize testing strategies to allow for meaningful comparisons of reported datasets from different investigators. If such a protocol can be agreed upon, then microperimetry promises to be a powerful tool for both researchers and clinicians alike. RP
REFERENCES
1. McClure ME, Hart PM, Jackson AJ, et al. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Ophthamol. 2000;84:244-250.
2. West SK, Munoz B, Rubin GS, et al. Function and impairment in a population-based study of older adults. The SEE project. Invest Ophthamol Vis Sci. 1997; 38:72-82.
3. Mangione CM, Gutierrez, PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthamol. 1999;128:45-53.
4. Scott IU, Schein OD, West S, et al. Functional status and quality of life measurements among ophthalmic patients. Arch Ophthamol. 1994;112:329-335.
5. Remky A, Lichtenburg K, Elsner AE, et al. Short wave automated perimetry in age related macular degeneration. Br J Ophthamol. 2001;85:1432-1436.
6. Hazel CA, Petre KL, Armstrong RA, Benson MT, Frost NA . Visual function and subjective quality of life compared in subjects with acquired macular disease. Invest Ophthamol Vis Sci. 2000;41:1309-1314.
7. Medina E, Degali AC, Blarzino MC, et al. Macular function impairment in eyes with early age related macular degeneration. Invest Ophthamol Vis Sci. 1997; 38:469-477.
8. Varano M, Scassa C. Scanning laser ophthalmoscope microperimetry. Semin Ophthalmol. 1998;13:203-209.
9. Fujii GJ, De Juan E, Humayun MS, et al. Characteristics of visual loss by scanning laser Ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularisation secondary to age related macular degeneration. Am J Ophthamol. 2003;136:1067-1078.
10. Mathew R, Pearce E, Sivaprasad S. Determinants of fixation in eyes with neovascular Age related macular degeneration treated with intravitreal ranibizumab. Am J Ophthamol. 2012;153:490-496.
11. Pearce E, Sivaprasad S, Chong V. Comparing fixation location and stability in patients with neovascular age-related macular degeneration treated with and without ranibizumab. Eye. 2011;25:149-153.
12. Carpineto P, Ciancaglini M, Antonio L, et al. Fundus microperimetry patterns of fixation in type 2 diabetic patients with diffuse macular oedema Retina. 2007;27:21-29.
13. Bacon J, Mody C, Acharya N, et al. To assess the pattern and location of fixation in patients undergoing treatment with intravitreal ranibizumab for exudative age related macular degeneration. Paper presented at: Annual congress of the Royal College of Ophthalmologists; Birmingham, United Kingdom; May 20-22, 2008.
14. Royal College of Ophthalmologists. Clinical guidelines. London. 2009. Available at: http://www.rcophth.ac.uk/page.asp?section=451§ionTitle= Clinical+Guidelines. Accessed July 30, 2012.
15. Seiple W, Rosen RB, Castro-Lima V, et al. The physics and psychophysics of microperimetry. Optom Vis Sci. 2012;89:1182-1191.
16. Acton J, Bartlett N, Greenstein V. Comparing the Nidek MP 1 and Humphrey Field analyser in normal subjects. Optom Vis Sci. 2011;88:1288-1297.
17. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135-160.
18. Squirrell DM, Mawer N, Mody CH, Brand CS. Visual outcome after intravitreal ranibizumab for wet age related macular degeneration: a comparison between: a comparison between best corrected visual acuity and microperimetry. Retina. 2010;30:436-442.
19. Seiple W, Rosen RB, Castro-Lima V, et al. The physics and psychophysics of microperimetry. Optom Vis Sci. 2012;89:1182-1191.
20. Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50:3464-3472.
21. Ehrlich, R, Mawer NP, Mody CH, et al Visual function following photodynamic therapy for central serous chorioretinopathy: a comparison of automated macular microperimetry versus best corrected visual acuity. Clin Exp Ophthalmol. 2012;40:e32-e39.
22. Parraano M, Oddone F, Tedeschi M, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab. Retina. 2010;30:1017-1024.
23. Meleth AD, Mettu P, Agron E, et al Changes in sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol. 2011; 52:1119-1126.
24. Midena E, Vujosevic S, Convento E, et al. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmist. 2007;91:1499-1503.
25. Cideciyan AV, Malgorzata S, Aleman TS. Macular function in macular degenerations: Repeatability of microperimetry as a potential outcome measure for ABCA4 associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012; 53:841-852.
26. Connolly EE, Beatty S, Loughman J, et al. Supplementation with all three macular carotenoids: response, stability and safety. Invest Ophthalmol Vis Sci. 2011; 52:9207-9217.
27. Ojima Y, Tsujikawa A, Hangai M, et al. Retinal sensitivity measured with the micro perimeter 1year after resolution of central serous chorioretinopathy. Am J Ophthalmol. 2008;146:77-84.
28. Senturk F, Karacorlu M, Ozdemir H, Karacorlu SA, Uysal O. Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011;151:303-309.
29. Fujita K, Yuzawa M, Mori R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: shortterm results. Retina. 2011 ;31:772-778.
30. Ozdemir H, Karacorlu SA, Senturk F, Karacorlu M, Ulysal O. Assessment of macular function by microperimetry in unilateral resolved central serous chorioretinopathy. Eye. 2008;22:204-208.
31. Oh J, Kim SW, Kwon SS, et al. Correlation of fundus autofluorescence Grey values with vision and microperimetry in resolved central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2012;53:179-184.
| David Squirrell, FRANZCO, is on the faculty of the Department of Ophthalmology at the University of Auckland in New Zealand. Rita Ehrlich, MD, is on the faculty of the Rabin Medical Center in Petah Tikva, Israel. Neither author reports any financial interest in any products mentioned in this article. Dr. Squirrell can be reached via e-mail at dsquirrell@adhb.govt.nz. |








